Prevalence of African Animal Trypanosomiasis among Wild Carnivores: A Systematic Review on the Global Distribution of Tsetse Fly Disease
Prevalence of African Animal Trypanosomiasis among Wild Carnivores: A Systematic Review on the Global Distribution of Tsetse Fly Disease
Ghulam Khadija Bashir1, Iqra Bashir2, Umer Ali3*
1. Department of Zoology, Wildlife and Fisheries University of Agriculture Faisalaba.
2. Institute of microbiology, Government college university Faisalabad.
3. Department of Biological Sciences, Tennessee State University, Nashville, TN 37209, USA.
*Correspondence to: Umer Ali, Department of Biological Sciences, Tennessee State University, Nashville, TN 37209, USA.
Copyright
© 2024 Umer Ali. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 04 July 2024
Published: 25 July 2024
ABSTRACT
This study looks at the prevalence of African animal trypanosomiasis (AAT) in populations of wild carnivores. African wildlife populations particularly those of wild carnivores are seriously threatened by African animal trypanosomiasis (AAT). It also evaluates existing control and preventative methods, identifies contributing factors, and assesses the disease's effects. In order to preserve biodiversity and the integrity of ecosystems, it highlights knowledge gaps, discusses research objectives, and formulates recommendations on how to effectively control and prevent AAT. It is essential for the health of animals and conservation efforts to comprehend the frequency of AAT in these carnivore species. Regional and temporal patterns of AAT incidence, evaluate diagnostic and treatment techniques, examine reservoir host dynamics, and assess the impact of AAT on carnivore populations through a thorough analysis of the body of research and field data gathering are analyzed in this review. This review demonstrates the wide prevalence of AAT in wild predators and its possible effects on ecosystem health and population dynamics. This research advances our knowledge of the prevalence of AAT in wild carnivores, which helps to preserve biodiversity and the integrity of ecosystems by managing these species in their native environment.
Keywords: Prevalence, AAT, carnivores, tsetse fly disease, Health
Prevalence of African Animal Trypanosomiasis among Wild Carnivores: A Systematic Review on the Global Distribution of Tsetse Fly Disease
Introduction
A group of wasting disease known as Animal Trypanosomiasis or Trypanosomoses are brought on by single-celled parasitic protozoans belonging to the genus Trypanosoma (order Kinetoplastida) (Giordani et al., 2016). Trypanosomiasis among African animals Tsetse fly disease (also referred to as tsetse disease) or African animal nagana is a transmissible disease that can affect both humans and animals (Steverding, 2008). It is one of the most common hemoparasitic illness affecting wildlife (Nantulya, 1990).
Trypanosoma vivax, Trypanosoma congolense, Trypanosoma brucei, Trypanosoma simiae and Trypanosoma suis are the many species of single-celled organisms responsible for a variety of parasitic disorders known as African Animal Trypanosomiasis or "Nagana." The bloodsucking tsetse fly Diptera (genus Glossina) is the cycle vector of AAT. Certain trypanosome species, including T. vivax, may also be actively spread via other biting flies, like Tabanus and Stomoxys species. AAT influence camels, hogs, horses and mules, ruminants and meat eaters, but animal trypanosomosis poses the greatest threat to rural farmers who raise livestock in sub-Saharan Africa (Diall et al., 2017).
T. congolense, T. vivax, and T. brucei spp. cause AAT. Different kinds of the disease are caused by two subspecies of T. brucei. Whereas T. b. rhodesiense typically produces a more acute infection and T. b. gambiense causes chronic infections. The parasites first appear in the blood, lymph, and peripheral organs in the first stage. In the second stage, they go to the central nervous system and cause serious neurological diseases. If therapy is not received, the disease is fatal (Büscher et al., 2017).
Trypanosoma brucei is a single-celled parasite that infects tsetse flies, is the cause of human African trypanosomiasis (HAT), commonly referred to as sleeping sickness (Büscher et al., 2017). There are three subspecies of it: T. b. brucei in animals, T. b. gambiense and T. b. rhodesiense in human beings (Jamonneau et al., 2019). Trypanosoma brucei gambiense is parasite that lives in a variety of environments including forests, mangroves, and savannah of West and Central Africa. It is also found in a variety of animal hosts most notably pigs. Trypanosoma brucei rhodesiense acts like a normal zoonotic parasite, primarily infecting cattle and wild mammals before occasionally spreading to people and only found in savannah regions of Eastern and Southern Africa (Steinmann et al., 2015).
Trypanosoma congolense is found in the bloodstreams of mammals (Gibson et al., 2017). It is the tiny parasite that is found in the bloodstream of ill goat , sheep and oxen (Gibson, 2003). Trypanosoma cruzi is found in Latin America and spread by the faeces of triatomine insects. It has a wide variety of natural hosts including armadillos, the raccoon, rabbits, native pigs, mice, and marsupial species. It also has a domesticated reservoir that includes dogs, cats, pigs, sheep, goats, cattle, and horses (Desquesnes et al., 2022). Trypanosoma vivix is known to be transmitted non-tsetse in some areas of Africa, including Ethiopia, Chad, and Sudan (Desquesnes et al., 2022). It lives and completes its brief life cycle in the proboscis of the insect. Because of its distinct behavior the parasite can be mechanically spread by other flies that feed on blood.(Giordani et al., 2016)
Trypanosoma simiae belongs to Nannomonas subgenus and causes fatal infections in pigs and result in death within one or two days after symptoms. Other domestic animals are also susceptible to this disease (Kasozi et al., 2022). Trypanosoma suis is a little-known parasite in Tanzania that was initially identified by Ochmann in 1905 as causing severe trypanosomiasis in pigs’ farms in Tanzania (Hutchinson and Gibson, 2015).
Some of these species of Trypanosoma are zoonotic. Zoonotic diseases are infectious illnesses that can spread from humans to vertebrate mammals through pathogenic agents such as bacteria, fungi, viruses, and prions. Zoonotic disease outbreaks are estimated to result in two billion cases of illness and more than two million fatalities annually, with far-reaching implications for public health. (Green et al., 2020) There are two types of the disease. The parasite Trypanosoma brucei gambiense is the cause of the one prevalent in West and Central Africa, whereas Trypanosoma brucei rhodesiense is the cause of the other one prevalent in East and Southern Africa (Dickie et al., 2020).
If there is a brief gap between the two meals to allow parasites to multiply in the insect mouthpart, tsetse flies can mechanically disseminate trypanosomes throughout mammals by beginning a blood meal on one infected host and finishing it on another (Bilala,b et al., 2024). Many domestic ungulates may harbour T. b. brucei however the condition is more severe in horses, dogs, and camels. In regions where multiple trypanosome species are present, cattle infections with mixed infections are common, and contemporary genetic techniques facilitate easier speciation. Numerous wild animal species in Africa are known to harbour at least one trypanosome species, making them potential reservoirs for highly contagious trypanosomes that can infect humans and livestock (Kasozi et al., 2022).
African trypanosomiasis is predicted to result in annual losses in agricultural gross domestic product of USD 4.7 billion, that has had a significant negative impact on agriculture and economic development in affected nations. About 37% of Zambia's land area is home to tsetse flies, and the reported cases of African animal trypanosomiasis (AAT) in cattle varies by region with estimates ranging from 1% to 90% (Afzal, M & Ali, U at el,.2024) The current Zambian government strategy of protecting natural resources and establishing state-protected National Parks and Game Management Areas has resulted in an increase in the population of tsetse flies, which spread the disease, and an expansion of wildlife populations that act as long-term reservoirs for African trypanosomiasis (Mulenga et al., 2020; Bilal et al., 2021).
Protozoans such as Trypanosoma brucei gambiense and Trypanosoma brucei rhodesiense can produce parasitic infections that are extremely dangerous to billions of people globally. The need for new drugs especially those made from plants, is growing as there are no effective immunisations and pharmaceutical resistance is increasing. Millions of people in Mexico, America and the United States of American Chagas disease and African trypanosomiasis, both of which are caused by T.cruzi and lack a vaccine or clinical research (Panda and Luyten, 2018).
Several species, including T. brucei, T. congolense, T. equiperdum, T. evansi, T. simiae, T. suis, and T. vivax, belongs to the genus Trypanosoma and cause diseases termed as trypanosomoses in a variety of animal hosts, including humans. Trypanosome outbreaks are common in Asia, Latin America, and Africa. Cattle, dromedary camels, goats, sheep, pigs, dogs, horses, donkeys, both domesticated and wild buffalo, warthogs, hippopotamus, reedbuck, waterbuck, antelope, giraffes, rhinoceroses, rodents, pangolins, primates, reptiles, and various wild ungulates and carnivores have all been reported to be carriers of Trypanosoma vivax (Fetene et al., 2021; Sajjad et al., 2024).
The animal disease surra is caused by T. brucei evansi and has an impact on a variety of mammals from various geographic locations. Cattle, camels, horses, and buffalos are the most affected species, although other animals, including wildlife, are also susceptible. Widespread throughout Africa, Asia, and Latin America, surra is spread non-cyclically by tabanids, other insects, vampire bats, and predators (Magri et al., 2021).
Wild animals are susceptible to trypanosomiasis especially lions because of the disease's intricate link to the host's immune system. T. b. gambiense and T. b. rhodesiense, two infecting trypanosome subspecies can evade immune responses, while people living in HAT-endemic regions build defences against trypanosome species. The illness affects both health and survival as it advances through two stages. It can have a typical duration of up to three years and be either acute or chronic (Venturelli et al., 2022).
T. b. brucei, the trypanosome subspecies that infects animals, is not very important economically for the cattle sector. However, because of its spread and effects on the economy, T. equiperdum is a significant threat. T. equiperdum is sexually transmitted and can result in large economic losses in several different parts of the world, unlike other fly borne trypanosomes. Although the immune system of mammals may regulate parasitemia to some extent, both innate and adaptive responses cannot offer total immunity against infection. Apolipoprotein L1 and other distinct innate immune pathways are essential for defence against trypanosome diseases like HAT. To counteract APOL1's effects, T. b. gambiense and T. b. rhodesiense have developed defence mechanisms. Furthermore, many Africans have mutations in APOL1 that provide resistance to T. b. rhodesiense infections, but they also raise the risk of chronic kidney disease (Pays et al., 2023; Noor et al., 2024).
Diseases can spread to humans and wild animals through wildlife farms, especially those that raise non-domesticated species for commercial gain. African lions are raised and bred on commercial farms in South Africa for a variety of purposes, such as hunting trophy, active tourism, and the export of bones for traditional medical items. There are chances for zoonotic sharing because of the lions and humans' high degree of direct connection. Infectious diseases like as trichinosis, filariasis, sarcoptic mange, pentastomiasis, echinococcosis, taeniasis, hepatozoonosis, anthrax, and babesiosis have been known to move from wild populations to captivity. The prevalence of lion-human contact and the risk of zoonotic disease transmission are increased by South Africa's extensive and intense breeding facilities (Green et al., 2020).
The family Trypanosomatidae is made up of a variety of protozoan parasites, some of which can infect more than one species of host. This family contains important human pathogens such as several species of Trypanosoma and Leishmania, which cause infections spread by insect vectors. Exploring endemic host-parasite interactions in Chile is intriguing due to its distinct biogeographic features and high endemism. But little is known about how trypanosomatids infect local species of vertebrates in Chile, particularly considering the arrival of foreign animals that bring with them their own parasite fauna. Trypanosoma cruzi, the most researched trypanosomatid in Chile, infects both native and foreign mammals using triatomine vectors; however, thorough assessments of T. cruzi infection in mammals as well as other trypanosomatids that may be present are absent. (Correa et al., 2020)
Companion animals are essential for the upkeep and dissemination of parasites, both zoonotic and veterinary. Their ranges have grown due to urbanisation and environmental changes, which has aided in the spread of parasites. However, it can be difficult to detect parasites because of their elusiveness and protected status. There are restrictions on the use of diagnostic procedures such as serological testing, macroscopic inspection, and molecular tools, and environmental conditions might affect the quality of samples (Rojas et al., 2024).
In the southern United States, the illness T. cruzi, which affects wild mammals, is harming conservation and peri domestic animals because of interaction with infected vectors. Studies on wildlife populations may not fully account for its effects but reports of Chagas disease in a rising number of exotic and zoo animals have implications for conservation and relocation initiatives. Making a diagnosis is difficult, and the list of illnesses could not fully reflect real cases (Busselman and Hamer, 2022).
Because of their restricted area and declining number, lions are the focus of African conservation efforts. For conservation attempts to be successful, it is essential to comprehend the natural surroundings of lions. By looking into lion habitat preferences and discovering common features in various circumstances, researchers may assess habitat suitability and direct conservation activities. In doing so, ecological variety is preserved (Sargent et al., 2022).
The ability of wild animals to suppress the growth of fatal African trypanosomes, which serves as the reservoir host for these parasites, is known as trypanotolerance. Trypanosomiasis is usually moderate in nature, but in captive populations breakouts can be lethal. Infested areas are primarily transmitted by tsetse flies, although there are alternative methods of transmission as well, including transmission through mechanical means via different biting flies and oral transmission by contaminated animals. The degree of infection varies according to environmental factors and pressure levels; coexistence is observed in independent wild animals but is interrupted by conditions such as famine, drought, and imprisonment. Trypanosomosis in captive and wild mammals is still little understood, despite its significance for conservation initiatives. This calls for more research and documenting of the clinico-pathology and processes of the parasitic infection (Mbaya et al., 2009).
Carnivores can contract trypanosomiasis by eating contaminated meat. The exposure of wildlife to trypanosomiasis is influenced by its diet, particularly wild Bovidae. Browsers are more vulnerable, but semi-browsers are more sensitive to attacks (Ali, U & Vungarala, S, et al,. 2024) Because of the lush forest, animals with greater infection rates include kudus, bushbucks, elands, and waterbucks. Wildlife hosts throughout the day are more susceptible, and incidences of infection are impacted by the movement of large animals (Kasozi et al., 2021).
African animal trypanosomiasis (AAT) is a disease that affects wild carnivore populations worldwide. The study aims to determine how common AAT is, how it affects wildlife health and conservation, what factors influence the disease's spread among wild carnivore species, how effective present management, diagnosis, and detection methods are, and what steps can be taken for preventing and controlling it to lessen its negative effects on wildlife.
The goals are to review the literature that has already been written about the prevalence and distribution of AAT in different wild carnivore species, to gather field data and samples to use diagnostic tools to assess AAT prevalence to analyze spatial and temporal patterns of AAT occurrence in relation to environmental factors, to investigate the effects on mortality, reproduction, and population dynamics, to investigate the role of wild carnivores as reservoir hosts, to determine knowledge gap.
Material and Methods
Study Design
Relevant documents were found by searching the ScienceDirect and PubMed (NCBI) databases. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria were followed in the conduct of this investigation to guarantee that every relevant piece of information was included in the review.
Search Strategy
A literature search was conducted using AJOL, PubMed Cross Ref, Research gate, and Google Scholar. 385 published publications on AAT and tsetse infections were subjected to meta-analysis. Eligibility was determined by study type, location, prevalence of tsetse species, overall distribution, lion /tsetse diagnosis method, and year of sampling. We visually searched through the reference lists of pertinent papers to find any studies that were missing.
Data inclusion
There were 132 studies in all. Every document released from 1990 to 2024 in English language was considered. Required parameters were searched out separately using some keywords like Prevalence, Trypanosom*, Control, tsetse, Glossina, Wild Carnivore, lion, AAT, and Primates. The published and unpublished literature, reports, and organization websites from pertinent institutions were then added to these data.
These sources included Google Scholar, the websites of the organizations, the references of the publications that were identified, and the key informants. This review was centred on AAT Prevalence and control. Nevertheless, in regions where cattle serve as a reservoir for HAT, a few Tsetse and Trypanosomiasis control actions that target both AAT and HAT were also included. Tanzania, south and west Africa, Brazil, Luangwa and Zambia, sub-Saharan Africa countries data was taken.
Data Quality
Peer-reviewed publications were given a particular significance when assessing the quality of the papers. The literature, surveillance reports from Google Scholar and other data sources follow an organized strategy to data collection, analysis, and interpretation, offering valuable insights into the global epidemiology, historical background, and regional dynamics of African trypanosomiasis in lion populations. Those articles whose relevance was not clear from the abstract alone were read full and concern piece of information collected from it. Articles that satisfied all above qualifying requirements were eventually included (Afzal et al., 2021).
For African Animal Trypanosomiasis (also known as "Nagana") and American Animal Trypanosomiasis (also known as "Chagas") we gathered previously published peer-reviewed articles and extracted pertinent data pertaining to AAT control and program-specific data, including outcome metrics. I investigated geographical variations of African trypanosomiasis in lion populations, as well as historical perspectives and worldwide epidemiology.
Data on global epidemiology were gathered from a variety of sources such as databases, government publications, and peer-reviewed publications. Studies with an emphasis on prevalence rates, geographic distribution, and related risk factors were chosen for inclusion in the review process due to their significance to lion populations. The global distribution of diseases was analyzed and visualized using descriptive statistics and spatial analytic techniques. Through the classification of areas according to geographical and ecological parameters, regional variations were investigated.
Results and Disscusion
Trypanosoma vivix
There are 39 African and Latin American nations where T. vivax, an African parasite, has been detected, together with 47 mammalian host species. T. vivax prevalence in tropical Africa varies from 5 to 15%, frequently accounting for as much as 50% of total trypanosome prevalence. T. vivax was identified in malnourished cattle for the first time in South America in French Guiana. Reports of the disease were then received from Venezuela, Guadeloupe, Martinique, Colombia, Suriname, Panama, Guyana, and Brazil. In some parts of South America, including the Venezuelan Llanos, the Brazilian Amazonia's lowlands, and the Pantanal wetlands of Bolivia and Brazil, T. vivax is endemic. T. vivax has been detected in Colombia. Cattle in Uganda have been shown to have a mixed T. vivax and T. congolense infection. Only cases of T. vivax in cattle and water buffalo have been reported in Central America. Cattle in Uganda have been shown to have a mixed T. vivax and T. congolense infection (Kapo et al., 2023).
Fig.1. Trypanosoma vivix Global distribution across the World (Kapo et al., 2023).
Trypanosma brucei
Cattle are linked in new areas and local outbreaks of sleeping sickness, which is primarily spread by domestic and wild animals. Via wild animal reservoirs, sporadic transmission to hunters and game park visitors happens. Although instances of foreign travel are uncommon, they do occasionally happen in the US. In 2020, fewer than 600 instances of sleeping sickness in Africa were documented, primarily due to Trypanosoma brucei gambiense. The identification of T. b. gambiense and T. b. rhodesiense in livestock species emphasises how crucial it is to include them in the fight against sleeping sickness (Kapo et al., 2023).
Fig.2. Trypanosoma brucei geographical distribution across the world
Trypanosoma congolense
Primarily present in African cattle, T. congolense is a parasite that causes illnesses in animals. It is a major contributor to serious and even deadly diseases in household animals, especially dogs. Less virulent strains induce persistent infections, while more virulent strains cause acute illnesses with significant fatality rates. Africa is home to several T. congolense species, including savannah, woodland, Tsavo, and Kilifi. There is evidence of travel-related infections in dogs, which implies that African dogs might be trypanotolerant. The Glossina morsitans tsetse fly is thought to be common in African cattle, which could aid in the transmission of T. congolense infection.
Fig.3. Trypanosoma congolense global distribution across the world (Kapo et al., 2023).
Trypanosoma evansi
T. evansi is the causative agent of Surra, a noncyclic parasitic disease that afflicts animals and results in a considerable death and morbidity rate. It is indigenous to Africa, the Middle and Far East, Asia, Mexico, Central and South America, and Europe. It is spread by tabanids, flies, vampire bats, or predators. The area north of Senegal to Kenya, which includes nations like Mauritania, Morocco, Algeria, Tunisia, Libya, Egypt, Sudan, Eritrea, and Ethiopia, is home to T. evansi. With occasional reports of epizootics from Argentina to Panama, it has spread throughout South America. T. evansi has recently been brought to France and Spain, where it is frequently found to cause mixed infections in household animals (Kapo et al., 2023)
Fig.4. Trypanosoma evansi global distribution across all over the World (Kapo et al., 2023).
Trypanosoma cruzi
Trypanosoma cruzi is the cause of Chagas disease, a serious international health concern that affects about 8 million individuals in 21 Latin American nations. The majority of cases of the disease are found in impoverished rural areas of Central and South America, where vectors are the main means of transmission. Although enzootic cycles of T. cruzi transmission have been found in a few southern states in the US, the proportion of locally acquired infections is still quite low. There are anywhere from 45,000 to 67,000 instances of Chagas disease in Spain alone, and there may be over 100,000 cases across the continent. Pets, including house cats and dogs, can serve as markers for infections in local populations of triatomines or in humans. Through recurrent contacts with infected vectors, wild animal species, including ocelots, raccoons, opossums, and rodents, are important players in the enzootic transmission of the parasite. T. cruzi may be traced to a larger family of trypanosomes that were first found in bats (Kapo et al., 2023).
Fig.5. Chagas disease's pattern of distribution in humans and animals, with light blue signifying non-endemic countries and dark blue suggesting endemic countries.
The frequency of AAT in Nigeria for 60 years. The analysis of variance's Tukey multiple pairwise comparison test reveals no discernible variation in the prevalence reports between decades (F (5, 68) = 1.616, P = 0.1676, r2 = 10.6%) (Fig. 6)
Fig 6. Prevalence of African Animal Trypanosomiasis in South Africa
T. vivax is the most phylogenetically different species of African trypanosome. Different isolates exhibit varying degrees of pathogenicity in cattle; in certain instances, they cause acute hemorrhagic infections, while in others, they cause chronic, subclinical infections. Though T. vivax, also known as T. congolense, has traditionally been thought to stay within the host's vascular system, certain strains may, particularly in late infections, also reach extravascular locations (such as lymph nodes, the eyes, and the cerebrospinal fluid), where they may cause direct harm to tissue and where they are more difficult to treat with medication Cortez et al., (2006)
Trypanosomiasis prevalence and taxonomic categories of wildlife hosts are correlated. It is highly prevalent in wildlife hosts belonging to the bovinae group in T. vivax and T. congolense. Their grazing habits expose them to biting flies such as tsetse, which is thought to be the source of infection (Fig. 7). Both wildlife hosts from the suidae family and those belonging to the Pantherinae group have an extremely high rate of mixed trypanosome infections (Kasozi et al., 2021).
Fig 7: Taxonomy of Wild Animal hosts and trypanosome species. Modified from Kasozi et al, (2021)
A third of the continent of Africa is afflicted by trypanosomiasis. The Tsetse people live over much of West, Eastern, Central, and Southern Africa. It displays the various tsetse species and subspecies. Tsetse populations need shade (47–49), high relative humidity (75–90%) with a slight saturation deficit, and moderate temperatures (23–25?). Trypanosomes and Tsetse are not able to survive at temperatures higher than 34.1? (Kasozi et al., 2021) (Fig. 8)
Fig 8. Trypanosomiasis-affected wildlife and Tsetse species' geographical distribution. Modified from (Kasozi et al., 2021)
Conclusion
Prevalence of AAT is high in Nigeria If concerted efforts are not made Nigeria may not be able to eradicate trypanosomiasis very soon due to tsetse infection. The incidence of AAT and tsetse infections in Nigeria has been extensively studied using microscopy; however, because of its sensitivity, PCR may provide a higher prevalence. To validate the results of research, study methodology and risk factor assessment are required. It is necessary to conduct additional research to determine how risk factor evaluation for AAT and tsetse infection might account for this difference. Trypanosomosis and free-living wild animals exist together in their natural environment, physical and somatic stress caused by storms, hunger, intercurrent infections, capturing, transfer, and captivity frequently compromised the animals' natural immunity to the infection.
References
1. Ali, U., Bilal, A., Iqbal, A., Ansari, M. S., Rakha, B. A., & Akhter, S. (2022). Ascorbic acid effect on frozen and thawed on sperm motility, plasma membrane integrity, livability and acrosome integrity of ring-necked pheasant (Phasianus colchicus) semen. Preprint
2. Ali, U., Vungarala, S., & Tiriveedhi, V. (2024). Genomic Features of Homologous Recombination Deficiency in Breast Cancer: Impact on Testing and Immunotherapy. Genes, 15(2), 162.
3. Büscher, P., Cecchi, V. Jamonneau and G. Priotto. 2017. Human african trypanosomiasis. Lancet 390(10110) : 2397-2409.
4. Busselman, R.E. and S.A. Hamer. 2022. Chagas disease ecology in the United States: recent advances in understanding Trypanosoma cruzi transmission among triatomines, wildlife, and domestic animals and a quantitative synthesis of vector–host interactions. Annu. Rev. Anim. Biosci. 10 : 325-348.
5. Bilal, A., Tanvir, F., Ahmad, S., Kanwal, N., Zulfiqar, H., & Ishaq, R. (2024). Pharmacokinetic Properties of Bioactive Compounds of Aloe vera against Pregnancy-Associated Plasma Protein A (PAPP-A) inducing Triple-Negative Breast Cancer. Kurdish Studies, 12(5), 157-168.
6. Correa, J.P., A. Bacigalupo, E. Yefi-Quinteros, G. Rojo, A. Solari, P.E. Cattan and C. Botto-Mahan. 2020. Trypanosomatid infections among vertebrates of Chile: a systematic review. Pathog. 9(8) : 661.
7. Desquesnes, M., M. Gonzatti, A. Sazmand, S. Thévenon, G. Bossard, A. Boulangé, G. Gimonneau, P. Truc, S. Herder and S. Ravel. 2022. A review on the diagnosis of animal trypanosomoses. Parasit. Vect. 15(1) : 64.
8. Diall, O., G. Cecchi, G. Wanda, R. Argilés-Herrero, M.J.B. Vreysen, G. Cattoli, G.J. Viljoen, R. Mattioli and J. Bouyer. 2017. Developing a progressive control pathway for African animal trypanosomosis. Trends Parasitolology 33(7) : 499-509.
9. Dickie, E.A., F. Giordani, M.K. Gould, P. Mäser, C. Burri, J.C. Mottram, S.P.S. Rao and M.P. Barrett. 2020. New drugs for human African trypanosomiasis: a twenty first century success story. Trop. Med. Infect. Dis. 5(1) : 29
10. Fetene, E., S. Leta, F. Regassa and P. Büscher. 2021. Global distribution, host range and prevalence of Trypanosoma vivax: a systematic review and meta-analysis. Parasit. Vect. 14 : 1-20.
11. Bilal, A., Iqbal, A., Rauf, A., Ali, A., & Azam, A. R. (2021). Top outbreaks of 21st century: a review. Palliat Med Care Int J, 4(2), 555632.
12. Gibson, W. 2003. Species concepts for trypanosomes: from morphological to molecular definitions? Kinetoplastid Biology Dis. 2 : 1-6.
13. Gibson, W., C. Kay and L. Peacock. 2017. Trypanosoma congolense: molecular toolkit and resources for studying a major livestock pathogen and model trypanosome. Adv. Parasitol. 98 : 283-309.
14. Giordani, F., L.J. Morrison, T.G. Rowan, H.P. De Koning and M.P. Barrett. 2016. The animal trypanosomiases and their chemotherapy: a review. Parasitol. 143(14) : 1862-1889.
15. Green, J., C. Jakins, E. Asfaw, N. Bruschi, A. Parker, L. de Waal and N. D’Cruze. 2020. African lions and zoonotic diseases: implications for commercial lion farms in South Africa. Anim. 10(9) : 1692.
16. Bilal, A., Tanvir, F., Ahmad, S., Shah, S. H. A., Ahmad, H. A., & Kanwal, N. (2024). Pre-clinical study of the bioactive compound Asiaticoside against the proteins inducing humn mammary carcinoma using molecular docking and ADME analysis. Remittances Review, 9(2), 3543-3576.
17. Hutchinson, R. and W. Gibson. 2015. Rediscovery of Trypanosoma (Pycnomonas) suis, a tsetse-transmitted trypanosome closely related to T. brucei. Infect. Genetic Evol. 36 : 381-388.
18. Jamonneau, V., P. Truc, P. Grébaut, S. Herder, S. Ravel, P. Solano and T. De Meeus. 2019. Trypanosoma brucei gambiense Group 2: the unusual suspect. Trends Parasitol. 35(12) : 983-995.
19. Kasozi, K.I., E.T. MacLeod, I. Ntulume and S.C. Welburn. 2022. An update on African trypanocide pharmaceutics and resistance. Front. Vet. Sci. 9 : 828111.
20. Kasozi, K.I., G. Zirintunda, F. Ssempijja, B. Buyinza, K.J. Alzahrani, K. Matama, H.N. Nakimbugwe, L. Alkazmi, D. Onanyang and P. Bogere. 2021. Epidemiology of trypanosomiasis in wildlife—implications for humans at the wildlife interface in Africa. Front. Vet. Sci. 8 : 621699.
21. Magri, A., R. Galuppi and M. Fioravanti. 2021. Autochthonous Trypanosoma spp. in European mammals: a brief journey amongst the neglected trypanosomes. Pathog. 10(3) : 334.
22. Mbaya, A.W., M.M. Aliyu and U.I. Ibrahim. 2009. The clinico-pathology and mechanisms of trypanosomosis in captive and free-living wild animals: a review. Vet. Res. Commun. 33 : 793-809.
23. Mulenga, G.M., L. Henning, K. Chilongo, C. Mubamba, B. Namangala and B. Gummow. 2020. Insights into the control and management of human and bovine african trypanosomiasis in Zambia between 2009 and 2019—a review. Trop. Med. Infect. Dis. 5(3) : 115.
24. M Afzal, U Ali, A Riaz, F Tanvir, A Bilal, S Ahmad - Journal of Population Therapeutics and Clinical …, 2024
25. Nantulya, V.M. 1990. Trypanosomiasis in domestic’altimals: the problems of diagnosis. Rev. Sci. Tech. Int. des Epizoot. 357-367.
26. Organization, W.H. 2013. Control and surveillance of human African trypanosomiasis: report of a WHO expert committee. World Health Organization.
27. Panda, S.K. and W. Luyten. 2018. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite 25.
28. Pays, E., M. Radwanska and S. Magez. 2023. The pathogenesis of african trypanosomiasis. Annu. Rev. Pathol. Mech. Dis. 18 : 19-45.
29. Noor, A., Bilal, A., & Ali, U. (2024). Towards Personalized Cancer Care: A Report of CRISPR-Cas9 Applications in Targeted Therapies and Precision Medicine. Journal of Health and Rehabilitation Research, 4(2), 1375-1380.
30. Rojas, A., N. Germitsch, S. Oren, A. Sazmand and G. Deak. 2024. Wildlife parasitology: sample collection and processing, diagnostic constraints, and methodological challenges in terrestrial carnivores. Parasitology Vect. 17(1) : 1-23.
31. Sajjad, M. K., Bilal, A., Iftikhar, A., Awais, M., Asif, I., Shaheen, F., & Zahoor, G. (2024). Examining the Association Between Pesticide Exposures and Chronic Diseases in Agricultural Workers. Remittances Review, 9(2), 2153-2176.
32. Sargent, R., N.J. Deere, P.J.K. McGowan, N. Bunnefeld and M. Pfeifer. 2022. Room to roam for African lions Panthera leo: a review of the key drivers of lion habitat use and implications for conservation. Mamm. Rev. 52(1) : 39-51.
33. Steinmann, P., C.M. Stone, C.S. Sutherland, M. Tanner and F. Tediosi. 2015. Contemporary and emerging strategies for eliminating human African trypanosomiasis due to Trypanosoma brucei gambiense. Trop. Med. Int. Heal. 20 : 707-718.
34. Steverding, D. 2008. The history of African trypanosomiasis. Parasitology Vect. 1 : 1-8.
35. Venturelli, A., L. Tagliazucchi, C. Lima, F. Venuti, G. Malpezzi, G.E. Magoulas, N. Santarem, T. Calogeropoulou, A. Cordeiro-da-Silva and M.P. Costi. 2022. Current treatments to control African trypanosomiasis and one health perspective. Microorganisms 10(7) : 1298.
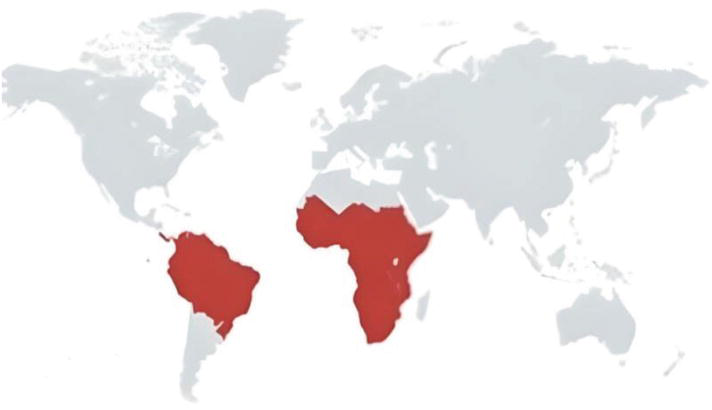
Figure 1
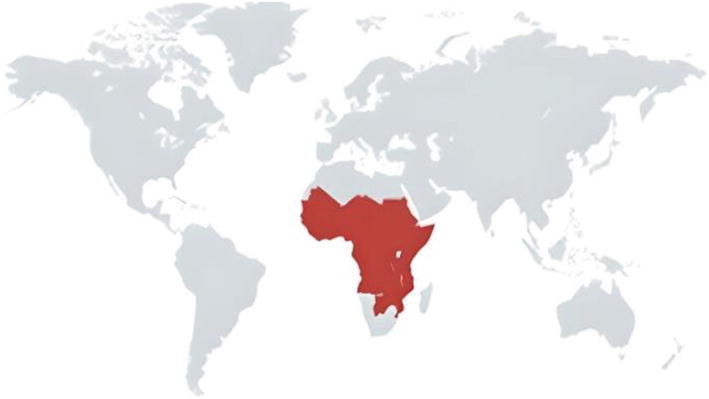
Figure 2
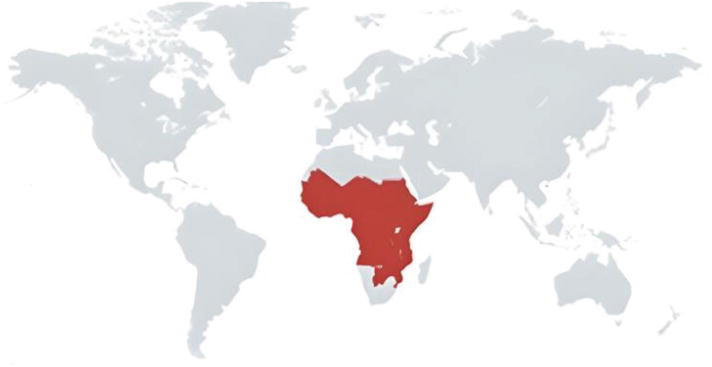
Figure 3
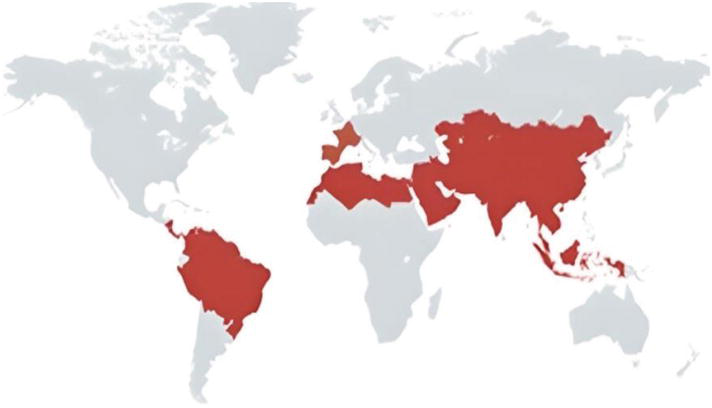
Figure 4
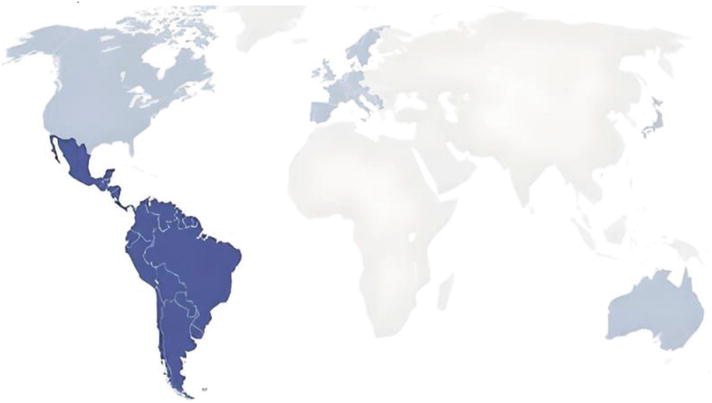
Figure 5
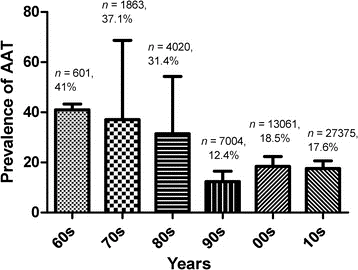
Figure 6
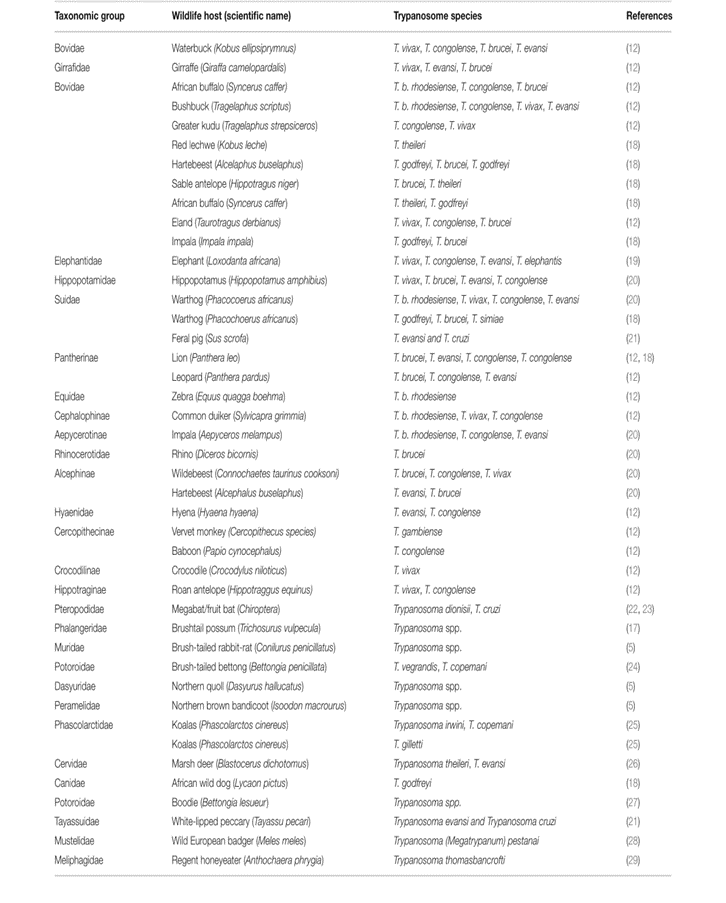
Figure 7
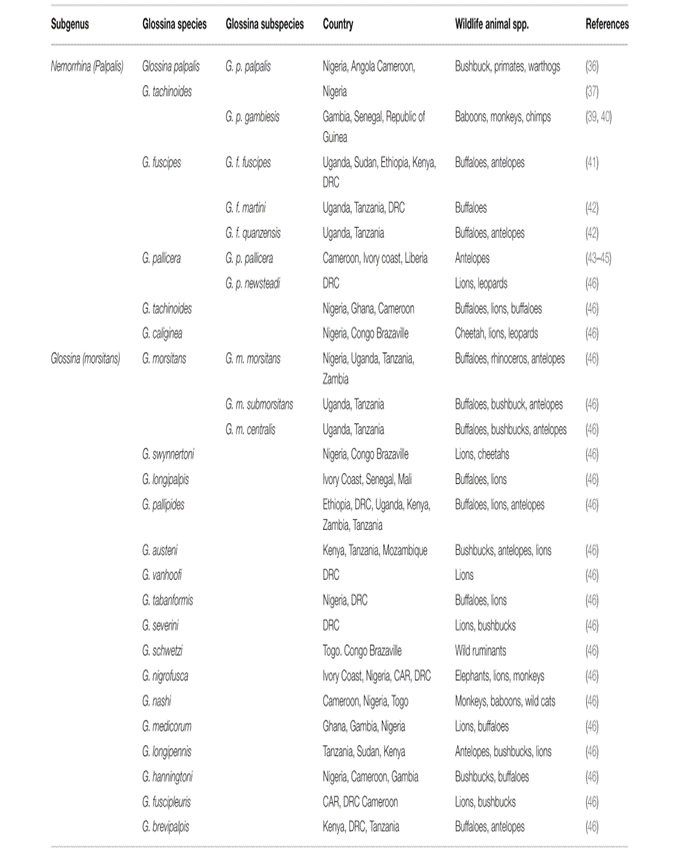
Figure 8
