Detecting and Treating Brain Metastases: Which Treatments are Best?
Detecting and Treating Brain Metastases: Which Treatments are Best?
Rithik Ostwal*, Sushil Panbude1, Neha Patel2, Dr Palanikumar Gunasekar 3, Ansari 4
1. Sushil Panbude, Consultant Radiologist, Ruby Hall Clinic, Pune, India.
2. Neha Patel, Additional Medical Director and Sr.Consultant Radiation Oncologist, India.
3. Dr Palanikumar Gunasekar, Consultant Radiation Oncology, Ashwin comprehensive cancer hospital, coimbatore, India.
4. Ansari, India.
*Correspondence to: Rithik Ostwal, MS in Cancer Molecular and Cellular Biology, United Kingdom.
Copyright.
© 2024 Rithik Ostwal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 28 August 2024
Published: 04 September 2024
Abstract
Background: Brain metastasis (BM) is the commonest intracranial neoplasm in adults. There are different treatment approaches for BM including whole-brain radiotherapy which is used mainly for patients with multiple lesions and those unfit for stereotactic radiotherapy (SRS) or surgery. Multiple prognostic indices have been developed for better patient selection for treatment and to identify patients with very short survival. We analyzed the survival for patients with brain metastasis and investigated some predictive factors for survival and we studied a small subset of patients with lung cancer without BM to look into some predictive factors for the development of BM.
Material and Methods: We retrospectively analyzed data of patients with radiologic diagnosis of brain metastasis who underwent whole-brain radiotherapy either alone or with other local treatment modalities (SRS or Surgery) at our institution, looking into overall survival and any predictive models for prognosis and we identified a subset of lung cancer patients without brain metastasis to try to find factors associated with the development of brain metastasis.
Results: The median overall survival in our study was 2.7 months and it was better for breast cancer (5.6 months) than lung cancer (3.5 months). In multivariate analysis, we found that the following factors remain the significant predictive factors for survival; use of local treatment modality (SRS or surgery), primary breast cancer, higher radiotherapy (RT) dose (30 Gy), controlled primary, age less than 65 years, female and 2 weeks or more interval from diagnosis of BM to the start of RT. In univariate analysis, we found from our study that, age > 65 years, female, smoking, weight loss, poor performance status, advanced stage at presentation and adenocarcinoma subtype were all factors associated with a higher incidence of BM in lung cancer patients. While in multivariate analysis, only age, smoking and weight loss remain risk factors for the development of BM in lung cancer patients.
Conclusion: The survival after whole-brain radiotherapy for BM is still poor. Our predictive models and other scoring systems have failed to identify the most important factors which can play a major role in the treatment decision. We think it is worthwhile to do more studies that focus on predictive models and to develop nomograms to predict BM in asymptomatic patients when the disease burden is low and effective local treatment whether SRS or resection could be used.
Keywords: brain metastasis, whole-brain radiotherapy, predictive models
Detecting and Treating Brain Metastases: Which Treatments are Best?
Introduction
The exact incidence of brain metastasis is unknown, and the range is wide. However, it is the commonest intracranial neoplasm in adults [1] with a frequency between 10-15%. [2] The incidence is higher in advanced stages and can reach up to 40% .[3]
Lung cancer is the commonest primary (36-40%) followed by breast cancer (15-25%), skin melanoma (5-20%) as sources of BM. less commonly it can occur in colon, rectal, renal and genitourinary cancers [4]. The median age of presentation is around 60 years [5] and this is related to the primary tumor site.[6]
Most patients have symptoms at diagnosis [7] although in some cancers, like small cell lung cancer, a high percentage of patients with BM are asymptomatic at initial diagnosis. [8]
Due to its availability, a computed tomography scan with contrast is the initial diagnostic modality in patients with suspected BM. [9] However, magnetic resonance is considered a better modality due to its high sensitivity and specificity in detecting small metastasis or metastasis in the posterior fossa.[10]
There are different treatment approaches for BM, including systemic treatment, steroids, whole brain radiotherapy (WBRT), SRS and surgery. The choice depends on the patient’s performance, age, status of primary, presence of extracranial disease, number of metastases and prior treatment.
The median survival with WBRT is quoted as being between 3.2-3.6 months[11] and 1.3 months with steroids .[12] The response rate for WBRT ranges from 40 to 60% [13-19]. Different fractionation schedules for whole brain radiotherapy (40Gy/15 fractions,30Gy/10 and 20Gy/5 ) have been used without a significant difference .[13]
Generally speaking prognosis is usually poor and estimated to be one month without treatment, prolonged to two months with steroids and 6 months with WBRT. However a small subset might survive more than one year. [20]
Accurate prognostic information is useful to optimize treatment for patients who may gain months of survival and to avoid overtreating patients who will derive little benefit. Many groups investigated different prognostic factors and tried to establish predictive models for survival. In one model, performance status (PS), age, extracranial metastases, and primary tumor status were crucial for survival [21], in another model, neurologic impairment at the time of diagnosis and the presence of multiple brain metastases were associated with significantly poorer survival, while solitary metastasis, gross total resection, and tumor histopathology of adenocarcinoma significantly prolonged survival. On the other hand primary tumor site, presence of active extracranial disease, and radiation dose had no significant effect on survival [22]. Kanefsky Index, radiation dose, solitary metastasis, and primary tumor size were good prognostic factors in a third model. [23]
Multiple prognostic indices have been developed, including Radiation Therapy Oncology Group Recursive Partitioning Analysis (RTOG RPA) [20] , the Rotterdam Score [24], the Scoring Index for Radiosurgery (SIR) [25], the Basic Score for Brain Metastases (BSBM) [26], the Golden Grading System (GGS) [27], The Rades classification (RADES)[28-29] , Graded Prognostic Assessment (GPA) [30] and the Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) for breast and lung .[31] These indices had included more or less the same prognostic factors, mainly PS, age, extracranial disease, site and status of the primary tumor, number of metastases and histologic subtypes.
Despite the availability of diverse scoring systems, there is still a lack of consensus regarding which clinical factors have the major impact on treatment decision-making concerning the use of WBRT in BM, especially in the light of the QUARTZ trial results [32] which showed a lack of survival benefit adding WBRT to dexamethasone in non-small cell lung cancer patients.
We have retrospectively analyzed data of patients with radiologic diagnosis of brain metastasis who underwent whole brain radiotherapy either alone or with other local treatment modalities (SRS or Surgery) at our institution, looking into overall survival and any predictive models for prognosis and we investigated a subset of lung cancer patients without brain metastases to find factors associated with the development of brain metastases.
Patients and Methods
This study retrospectively reviewed the records of patients with a radiologic diagnosis of brain metastasis who underwent whole-brain radiotherapy either alone or with other local treatment modalities (SRS or Surgery) at our institution. Patients with a diagnosis of leukemia, lymphoma and unknown primary were excluded. Information regarding patient characteristics, disease characteristics, treatment and survival were collected.
Patient data included age, gender, smoking, PFS, weight loss and presentation of BM. Disease variables included , primary tumor , histopathologic type ,number and size of BM, method of diagnosis of BM, the status of primary and its stage at initial presentations and date of diagnosis of both the primary and BM. Treatment variables included WBRT,SRS, metastasectomy , use of systemic treatment either before or after diagnosis of BM and WBRT dose.
Another cohort was patients with lung cancer without brain metastasis and their data were compared with a similar group with brain metastasis to determine predictive factors for the development of brain metastasis.
Univariate and multivariate analyses were performed to determine the impact of the following parameters on overall survival: age, gender, Eastern Cooperative Oncology Group performance status, primary tumor, the status of primary tumor control, number of intracranial metastases, different radiotherapy fractionations, time from diagnosis of the primary and development of brain metastasis.
Univariate analysis consisted of Fisher’s exact tests, with the factors achieving statistical significance (defined as P< 0.05 throughout this study in two-sided tests) entered into the multivariate analysis (Cox proportional hazards model).Actuarial survival curves were calculated by the Kaplan–Meier method, and differences were compared using the log-rank test. Overall survival (OS) was defined as the length of time from the initiation of WBRT to death or to the last follow-up date.
Objectives:
Primary outcome was to determine overall survival in patients with BM after whole brain radiotherapy.
Secondary outcome was to identify some predictive factors for survival, the level of concordance between CT and MRI brain and to identify predictive factors for development of brain metastasis in lung cancer patients cohort.
Ethical considerations: This study is retrospective, all data were deidentified and most patients were expired by the time of analysis, for all those factors, this study was exempted from IRB approval.
Results
This study was conducted on 203 patients with radiologic diagnosis of brain metastasis who underwent whole brain radiotherapy either alone or with other local treatment modalities (SRS or Surgery).
Table (1): General characteristics of the studied patients
|
|
|
Number |
Percent |
|
Age (years) |
Mean + SD |
62.85 ± 12.26 |
|
|
|
Range |
27-87 |
|
|
Age subgroups |
<65 years |
102 |
50.2 |
|
|
>65 years |
101 |
49.8 |
|
Gender |
Female |
116 |
57.1 |
|
|
Male |
87 |
42.9 |
|
Smoking |
Yes |
99 |
48.8 |
|
|
No |
|
51.2 |
|
Weight loss |
Yes |
25 |
12.3 |
|
|
No |
178 |
87.7 |
|
KPS |
<70% |
70 |
34.5 |
|
|
>70% |
133 |
65.5 |
|
Primary tumour site |
Lung |
101 |
49.8 |
|
|
Breast |
45 |
22.2 |
|
|
Skin melanoma |
16 |
7.9 |
|
|
GIT-oesophagus |
3 |
1.5 |
|
|
GIT-colon |
8 |
3.9 |
|
|
GIT-rectum |
7 |
3.4 |
|
|
GIT-anal canal |
1 |
0.5 |
|
|
Genitourinary kidney |
9 |
4.4 |
|
|
Genitourinary bladder |
1 |
0.5 |
|
|
Genitourinary prostate |
2 |
1.0 |
|
|
Gynaecological |
7 |
3.4 |
|
|
Other |
3 |
1.5 |
The age ranged from 27 – 87 years with mean ± SD of 62.85 ± 12.26. The studied group included 116 females (57.1%) and 87 males (42.9 %). The commonest primary was the lung (49.8%) and the commonest pathologic subtype was adenocarcinoma (43.3%). Half of the patients presented with stage 1V, and in 80% the primary tumor was uncontrolled and was associated with extracranial metastases. The majority had 2 or more brain lesions (70%) and most of the patients were symptomatic at presentation (88%). CT brain was the only imaging modality in 76% and the concordance between CT and MRI brain for those who had the two modalities was 50%. The vast majority were treated with whole brain radiotherapy alone (96%), two thirds received radiotherapy 2 weeks or more after initial BM diagnosis and twenty Gray regimen was utilized in 80% of patients, as shown in Table 1.
Table 2: Relationship between primary tumour site and the interval from diagnosis of primary and the diagnosis of Brain Metastasis (BM)
|
|
|
Number |
Percent |
|
Age (years) |
Mean + SD |
62.85 ± 12.26 |
|
|
|
Range |
27-87 |
|
|
|
|
|
|
|
Age subgroups |
<65 years |
102 |
50.2 |
|
|
>65 years |
101 |
49.8 |
|
|
|
|
|
|
Gender |
Female |
116 |
57.1 |
|
|
Male |
87 |
42.9 |
|
|
|
|
|
|
Smoking |
Yes |
99 |
48.8 |
|
|
No |
|
51.2 |
|
|
|
|
|
|
Weight loss |
Yes |
25 |
12.3 |
|
|
No |
178 |
87.7 |
|
KPS |
<70% |
70 |
34.5 |
|
|
>70% |
133 |
65.5 |
|
Primary tumour site |
Lung |
101 |
49.8 |
|
|
Breast |
45 |
22.2 |
|
|
Skin melanoma |
16 |
7.9 |
|
|
GIT-oesophagus |
3 |
1.5 |
|
|
GIT-colon |
8 |
3.9 |
|
|
GIT-rectum |
7 |
3.4 |
|
|
GIT-anal canal |
1 |
0.5 |
|
|
Genitourinary kidney |
9 |
4.4 |
|
|
Genitourinary bladder |
1 |
0.5 |
|
|
Genitourinary prostate |
2 |
1.0 |
|
|
Gynaecological |
7 |
3.4 |
|
|
Other |
3 |
1.5 |
One way ANOVA test is used.P1 equals breast VS lung.P2 equals breast VS others.P3 equals lung VS others. The significance level is 0.05.
Breast cancer needs a longer interval before the development of BM ,compared to very short interval for lung cancer ( with mean of 69 months versus 5.4 months respectively), as illustrated in Table 2 .
The survival
The survival was very poor and about 97% of patients expired at the time of data analysis, as shown in Table 3 and the median survival was 2.7 months as shown in figure 1
Table 3: Fate of the studied group (n = 203)
|
|
Number |
Percentage |
|
Alive |
7 |
3.4 |
|
Dead |
196 |
96.6 |
Figure (1): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy
The median overall survival in patients with BM after whole brain radiotherapy in this study { (95% confidence interval level: 70.9-91.1 days (2.4-3 months)},as shown in figure 1.
Factors influencing survival:
The survival was significantly better in the following groups: patients<65 versus≥65,females,primary breast, with earlier stage, absence of extracranial metastases, with 1-2 brain lesions, treated 2 weeks or more after diagnosis of BM, treated with combined modality versus whole brain alone and those treated with 30 Gys over 20 Gys. While the following parameters did not influence the survival: KPS presence or absence of symptoms and imaging modality, as illustrated in table 4 and figures 2-6
Table 4: Relationship between survival and various demographic and clinical factors
|
|
Mean |
Std. Dev. |
Median |
Q1 |
Q3 |
P-value |
||
|
Age (yrs) |
< 65 |
248 |
340 |
138 |
71 |
270 |
0.028 |
|
|
>65 |
152 |
225 |
92 |
50 |
159 |
|||
|
Gender |
Female |
245 |
339 |
138 |
71 |
249 |
0.018 |
|
|
Male |
141 |
203 |
93 |
53 |
143 |
|||
|
KPS |
> 70 |
211 |
238 |
143 |
70 |
232 |
0.079 |
|
|
< 70 |
195 |
317 |
97 |
55 |
176 |
|||
|
Primary Tumour |
Breast |
314 |
408 |
169 |
80 |
285 |
0.007 |
|
|
Lung |
192 |
270 |
105 |
71 |
186 |
|||
|
Other |
124 |
169 |
79 |
55 |
115 |
|||
|
Stage |
I |
552 |
794 |
193 |
128 |
977 |
0.059 |
|
|
II |
233 |
298 |
116 |
67 |
276 |
|||
|
III |
157 |
258 |
87 |
63 |
148 |
|||
|
IV |
198 |
269 |
100 |
65 |
220 |
|||
|
Extracranial metastases |
No |
545.7 0 |
590.437 |
324 |
81 |
973 |
0.08 |
|
|
Yes |
176.1 8 |
248.924 |
98 |
64 |
186 |
|||
|
Unknown |
|
|
147 |
47 |
245 |
|||
|
Stage IV at presentation |
No |
203.1 2 |
314.752 |
99 |
65 |
200 |
0.916 |
|
|
Yes |
198.4 8 |
268.720 |
100 |
65 |
220 |
|||
|
Number of Brain Metastases |
1 (61) |
228.3 |
364 |
117 |
66 |
241 |
0.016 |
|
|
2 (27) |
269.4 |
251 |
94 |
65 |
173 |
|||
|
>2 (115) |
751 |
55 |
64 |
44 |
121 |
|||
|
Symptoms |
No |
201 |
302 |
127 |
55 |
201 |
0.994 |
|
|
Yes |
201 |
292 |
99 |
66 |
215 |
|||
|
BM diagnosis |
CT + MRI |
207 |
287 |
115 |
72 |
170 |
0.286 |
|
|
CT |
185 |
264 |
98 |
64 |
212 |
|||
|
MRI |
290 |
428 |
133 |
69 |
245 |
|||
|
Interval from BM diagnosis to RT start |
< 2 weeks |
124.4 6 |
117.927 |
87 |
44 |
141 |
0.014 |
|
|
> 2 weeks |
238.1 0 |
341.317 |
115 |
69 |
223 |
|||
|
Treatment modality |
SBRT+WBRT |
302 |
|
302 |
30 2 |
302 |
<0.001 |
|
|
Surgery + WBRT |
892 |
676 |
586 |
22 3 |
1741 |
|||
|
WBRT |
151.1 |
230 |
81 |
64 |
174 |
|||
|
RT Dose |
20Gy in 5# |
174 |
280 |
87 |
59 |
166 |
0.013 |
|
|
30Gy in 10# |
308 |
319 |
191 |
12 9 |
299 |
|||
Independent T-test. One way ANOVA. Significance level is 0.05
Figure (2): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy in relation to the primary tumor.
Figure (3): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy in relation to the histopathology of the primary tumor.
Figure (4): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy in relation to the number of BM
Figure (5): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy in relation to the treatment modality of BM
Figure (6): Kaplan-Meier survival plot of the studied patients with brain metastases demonstrating the overall survival after whole brain radiotherapy in relation to the radiotherapy dose.
Predictive factors for survival:
In multivariate analysis, The use of local treatment modality (SRS or surgery),primary breast cancer, higher RT dose 30 Gy), controlled primary, age less than 65 years ,female and 2 weeks or more interval from BM to start RT remain the significant predictive factors for survival as shown in Table 5
Table 5: Survival analysis (Kaplan Meier curve for predictive model)
Factors associated with development of brain metastasis in lung cancer patients:
There is a significant relationship between the occurrence of brain metastasis in lung cancer patients with the following factors; older age ( ≥ 65 y) , females, smoking, weight loss, ECOG, advanced stage and adenocarcinoma subtype, as shown in Table 6.
Table 6: Relationship between the occurrence of Brain Metastases and the studied parameters among lung cancer patients (n= 96)
Multivariate analysis for predictive factors for development of brain metastasis in lung cancer:
Age, smoking, and weight loss were the independent risk factors for occurrence of brain metastasis among lung cancer patients with odds ratio (2.80, 4.31 and 5.34 respectively) and p values: 0.031,
0.031 and <0.0001, respectively, as illustrated in Table 7.
Table 7: Binary logistic regression analysis (inter method) for independent risk factors for occurrence of brain metastasis among lung cancer patients (n=96).
|
|
S.E. |
Wald X2 |
P Value |
Odds ratio |
95% CI. |
|
|
Lower |
Upper |
|||||
|
Age |
1.30 |
4.68 |
0.031 |
2.80 |
1.3 |
208.74 |
|
Sex |
1.07 |
0.03 |
0.858 |
0.19 |
0.148 |
9.90 |
|
Smoking |
1.74 |
6.12 |
0.013 |
4.31 |
2.45 |
2279.04 |
|
Weight loss |
1.48 |
12.99 |
<0.0001 |
5.34 |
11.41 |
3791.38 |
|
ECOG |
40193.08 |
7.81 |
0.099 |
17.92 |
- |
- |
|
Stage of primary tumour |
1.134 |
4.80 |
0.187 |
2.26 |
0.011 |
0.963 |
|
Tumour histopathology |
22887.01 |
0.019 |
0.999 |
22.124 |
- |
- |
Discussion
Our study included 2 cohorts of patients, one with brain metastasis from a variety of primary cancers and another cohort of lung cancer patients without brain metastasis. Our department is a tertiary radiotherapy referral center, and our patients are referred from other disciplines for radiotherapy, so we cannot comment on the true incidence of BM in our patient population. Half of our patients were aged 65 years or more which agrees with most trails, which quoted a median age of 60 years. [5,33-34]
The effect of gender on BM incidence is unclear, some reported higher incidence in females [35], others reported higher incidence in males[36], while some others did not report any impact .[37] We found that BM was higher in women than men, but this could be due to inclusion of more female patients in our study; about 22% of our patients were diagnosed with breast cancer.
In our cohort, BM was more frequent among lung cancer patients compared with breast cancer and melanoma patients (50%,22% and 8% respectively), which is concordant with other studies [15.22], which reported an incidence of 56% and 57.5% for lung cancer, while others reported an incidence of 50 % and 20 % for lung and breast cancers respectively. [38,31] A few trials reported higher incidence in breast cancer than lung cancer, but the majority of their patient population were females .[39]
Adenocarcinoma is the commonest pathologic subtype that metastasizes to the brain according to many studies [40,41] and this was confirmed in our study (43%) and in another study which reported 46% .[22]
BM is more common in advanced stages , and it was 50% in our patients and 40 % in others studies. [3,35,42] Half of the patients in our cohort presented with stage 1V cancer, which is alarming and needs an intervention like adoption of screening programs specially for lung cancer.
Some studies have reported a higher incidence of BM in the presence of extracranial metastases. [43] We found about 80 % of our patient had extracranial disease which is comparable to incidences of 85 %[44] and 60%[45] reported in two other studies. We think that the presence of extracranial metastases at the initial diagnosis or its development during the course of disease, is a clear risk factor for the development of brain metastasis.
Most patients present with multiple lesions as documented in our cohort (70%) and others [46-48,1], while some authors have reported higher incidence of solitary metastasis. [49-50]
Seventy five percent of our patients had CT scan as their only investigation for BM. It was noted that patients who had both CT and MRI, the concordance rate was only 52%. forty percent of our patients with a solitary lesion on CT scan, were found to have multiple lesions on MRI, which is in agreement with some studies [4], but lower than others which reported 80%. [51] It is worthwhile to mention that in 20 % of our cases, CT scan was negative while MRI was positive for brain metastasis. We think it is an acceptable practice to start with CT brain with contrast if BM is suspected, however MRI should be considered if CT scan is negative, in the face of a high level of suspicion, as well as for exclusion of BM in certain high risk cancers, such as lung cancer.
Ninety percent of our patients were symptomatic at time of diagnosis, which in agreement with Tsakonas, et al [7] who reported 93% and Cairncross, et al.[52] who reported 66% in their patients. Only trials utilized MRI reported higher percentage of asymptomatic patients like Hjorthaug, et al.[53] who detected BM in 50 % of asymptomatic patients.
The median size of BM was 1 cm in our patients; however, some trials reported a larger median size of 2.1 cm .[43] Whole brain radiotherapy as a sole modality was utilized in 96% of our patients , although 30 % of them presented with a solitary metastasis and this highlights the underutilization of local treatment whether surgery or SRS. Most of our patients treated with 20 Gy over 5 fractions (80%).
Breast cancer typically shows a longer interval before the development of BM compared with relatively very short interval for lung cancer. In our group, the mean duration was 69 months for breast cancer versus 5.4 months for lung. An interval of 2.6-7 months for the lung was reported in some studies [35.54-55] and an interval of 39-47 months for the breast in other studies . [56-58]
The survival of patients with brain metastasis , especially If multiple, is poor , regardless the treatment modality used. In our study ,97 % of our patients demised, while only 3% were alive when censored to last follow up at the time of data collection. In a group of more than 900 patients with BM ,Wong, et al.[59] reported that 94% of their patients were deceased.
The median overall survival for our patients was 2.7 months which was identical to Wong, et al.[59] and similar to Periestman, et al. [60] who reported 2.6 months. While Fleckenstein, et al[6] Lagerwaard et al.[24] and Silva et al[26] reported a higher median survival of 3.8 months and 3.4 and 4.5 months respectively. On the other hand, Chan et al[6] reported a shorter median survival of 2.3 months. Although there are some differences in the median survival among different trials, for those short- lived patient cohort , the direct comparison between different trails, considering the heterogeneity of the patient populations and different methods for survival calculation( calculated form date of diagnosis versus date of treatment), is difficult, although it did confirm a poor survival.
In our study and in that of Wong, et al [59] patients ≥ 65 years had a poorer survival compared with a younger group, while Jakhar, et al[64] failed to show any impact of age on the survival . When we categorized patients into≤ 60 years and > 60 years, the difference in survival was highly significant and this coincide with Jeene et al. [65] who reported a significant difference between different age groups (<50 ,50-59,60-69 and≥ 70 years).
Females had a better survival than males as documented in our study and in others [59,65] ,while other studies did not demonstrate any gender difference .[64-66] Performance status did not affect the survival in our patients and this is in accordance with some studies [66,64] while in contradiction to others [67-68] who reported a better survival among patients with good performance status.
In our cohort, the survival was significantly better for patients with breast rather than lung cancer (median survival 5.6 months and 3.5 months respectively), and it was lowest in skin cancer where melanoma was the predominant subtype. A median survival of 8 months for breast cancer patients was reported in one study [57] and a median survival of 2.7- 6.3 months for lung cancer patients reported in some studies .[35,69-70] Jeene et al [65] reported a median survival for the breast and lung cancer patients of 3.7 months and 2.7 months respectively and Wong et al [59] reported a median OS of 4.3 months , 2.2 months , 2.1 months and 2.7months, in breast , GIT, GU and lung cancers patients respectively.
In our study there was a marginally significant survival advantage for those presented with early stage compared to those presented with advanced stage.
We have found that the presence of extracranial disease was associated with a poor survival .In a study by Wang, et al [71] for non-small cell lung cancer patients with brain metastases ,not just the presence or absence of extracranial disease has affected the survival, but the number of extracranial disease has also influenced outcome as well. This result was reproduced by Gerdan, et al. in a similar cohort with non small cell lung cancer and second cohort of small cell lung cancer patients and also in a third cohort of patients with breast cancers. [72-74]
In our cohort, patients presented with a fewer number of brain metastases (1-2) had a better survival when compared with patients with multiple brain metastases and this is in agreement with most publications [75.76,66,31], while Fleckenstein, et al.[61] was unable to demonstrate any significant difference. Although 48% of our patient cohort had 1-2 lesions, only 4 % had local treatment (surgery or SRS) which highlights underutilization of local treatment modalities.
Patients who are asymptomatic from brain metastases is associated with a better survival when compared with symptomatic counterparts as reported in most trials[77,80] , however we found the survival was not adversely affected with symptoms and this could be due to the fact that almost 90 % of our patient cohort were symptomatic at time of diagnosis .
Brain metastasis imaging modality whether MRI or CT scan did not affect the survival as per our study, however we cannot make any conclusion as the number of patients who had MRI was low.
We found the survival was better if the interval between BM diagnosis and initiation of radiotherapy was more than 2 weeks rather than less 2 weeks . Mehta, et al. [81] and Hansen et al.[82] did not show any impact and Nieder et al. [83] concurred except in those patients without extracranial disease where the survival was better if this interval was less than 2 weeks compared to poorer survival if it was more than 2 weeks. .
In our study the median survival was 19 months,10 months and 2.7 months for S+WBRT,SRS+WBRT and WBRT alone, respectively and this was statistically significant . Lentzsch, et al.[23] reported a median OS of 20 months,6.5 months and 1.25 respectively for patients treated with surgery, WBRT or steroids and Ekici et al.[34] reported a median survival of 13.5 months for S+WBRT and 5.5 months only for WBRT alone, while
Hazuka et al.[22] reported 11 months median survival with S+WBRT. There is general agreement in most trials that the inclusion of a local treatment modality is associated with a better survival.
It is unclear if higher doses of WBRT would improve the survival or not. Twenty Grays in 5 fractions achieved similar survival as 30 Gy in 10 fractions, with a better toxicity profile, as reported in 2 studies . [84,131] However, we found 30 Gy was associated with a significantly better survival when compared with 20 Gy and this is in agreement with Wong et al.[59] and Thakur et al . [66]
In our study, adenocarcinoma of the lung was associated with a significantly poorer survival when compared to non adenocarcinoma and this was reported by Harada et al .[85] We also found that the survival was significantly better for infiltrating ductal carcinoma of the breast when compared with lung cancer; both non small and small cell cancer, and this was in agreement with Jeene et al.[65] and Wong et al [59].Other studies have found no prognostic significance of histology .[88.87]
Tumor size did not seem to influence the survival in our group, and this is concordant with Staudt el al.[88] who reported no significant effect of the size on survival.
Thakur et al.[66] on the other hand, reported a better survival for lesions < 3cm when compared with
those ≥3 cm.
In multivariate analysis, we found that the use of a local treatment modality (SRS or surgery),primary breast cancer, higher RT dose (30 Gy), controlled primary, age less than 65 years ,female and 2 weeks or more interval from BM to start RT remain the most significant predictive factors for survival. In a study for survival among breast and lung cancer patients treated with WBRT by Jeene et al.[65] they reported that primary site ,age and sex were the predictive factors. According to Wong et al. [59] primary tumor site still stands as a significant predictive factor in addition to age and KPS, while Thakur et al.[66] reported that number and size of BM in addition to RT dose were the most predictive factors for survival. Patil et al.[89] reported that number of BM and KPS were significant predictive factors , while Saito et al.[90] found that high KPS and resection status were the significant predictive factors for survival. It is therefore evident from trials that have been conducted, that there is no consistency demonstrable among the predictive factors for the survival.
Many scoring systems or predictive models for better selection of patients have been assessed. In 1997 The Radiation Therapy Oncology Group (RTOG) developed the Recursive Partitioning Analysis (RPA)[20] and the Grading Prognostic Assessment (GPA) was developed in 2008 [91] and more recently, disease specific GPAs were developed mainly for lung and breast in 2012 . [70] RTOG RPA classified patients into 3 classes; Class One, those who have Karnofsky performance score (KPS) of ≥70, age <65, and controlled primary tumour without extracranial metastases, Class 3 patients have KPS <70, all other patients fall into Class 2, including those with KPS ≥70 but other unfavorable characteristics, such as uncontrolled primary tumor, extracranial metastases, or age ≥65. The median survival for classes 1,2 and 3 were 7.1,4.2 and 2.3 months respectively . [20,43,92] GPA classification excluded the status of extracranial disease acknowledging only its presence or absence; it kept age and KPS and added number of brain metastases and each factor was given values of 0,0.5 and 1. Four prognostic groups were created and the median survival was 2.6 months,3.8 months, 6.9 months and 11 months for GPA scores of 0-1,1.5-2.5,4 and 3.5-4 respectively. [91]
Diagnosis-specific graded prognostic assessment was developed after the primary tumour site was shown to be an important prognostic factor in some studies. [30]
Despite the availability of diverse scoring systems, there is still a lack of consensus regarding which clinical factors have the major impact on treatment decision-making concerning the use of WBRT and making a clinical decision based on them is still a challenge as it is difficult to identify patients with very short survival (< 2 months) after WBRT and the survival for groups with poor prognostic score based on RPA OR DS GPA is still heterogonous.
Quartz trial which is the only randomized trial that investigated the benefit of WBRT in non small lung cancer patients with multiple brain metastases. In this trail, patients were prospectively randomized into best supportive care including dexamethasone plus WBRT or best supportive care and dexamethasone alone .This trail confirmed that WBRT does not significantly improve quality of life or overall survival, except for young patients . [32]
Although this trail was criticized in relation to its protracted recruitment period and its inclusion of patients with multiple poor prognostic factors, yet it still quires the role of WBRT in non small cell lung cancer.
In an attempt to find predictive factors for the development of BM among lung cancer patients, we collected data for 48 patients with lung cancer without BM and compared with same number of patients from our study group with BM. In univariate analysis, we found from our study that, age > 65 years, female ,smoking, weight loss, poor performance status, advanced stage at presentation and adenocarcinoma subtype were all factors associated with higher incidence of BM in lung cancer patients. While with multivariate analysis , only age, smoking and weight loss remain risk factors for development of BM in lung cancer patients. Different studies have investigated alternative predictive factors for development of BM in lung cancer patients. In a study by Waqar et al.[93] it was found, on multivariate analysis, that younger age, adenocarcinoma or large cell histology, tumor size > 3 cm, , tumor grade ≥ II and node positive disease were all factors associated with brain metastases and they created a scoring system to predict the development of BM.
Zhang et al.[94] managed to gather data for 26, 154 patients with lung squamous cell carcinoma. After doing multivariate cox regression, they found that age at the time of diagnosis, tumor grade and stage, the number of extracranial metastatic sites, the use of chemotherapy, surgery, and radiation were independent factors for predicting BM. Then they developed a nomogram using those factors to predict BM.
In an interesting study by Yokoi et al.[95] who investigated the value of intensive follow up with brain CT scan in early-stage lung cancer patients treated with surgery, they detected BM in 11/128 patients, of which 7/11 were asymptomatic and 5/11 had a single metastatic lesion. Even in this group with early lung cancer about 9% developed brain metastasis and the majority were asymptomatic ,so it is probably reasonable to expect a higher incidence of BM among asymptomatic patients who presented with advanced disease.
Conclusion
The role of whole brain radiotherapy in the management of multiple brain metastases is still a challenge. Despite the availability of diverse prognostic scoring systems, there is still a lack of consensus regarding which clinical factors have the major impact on treatment decision-making. However, it is reasonable not to offer WBRT for patients with multiple brain metastases, if the patients are elderly, have poor performance status, uncontrolled primary and have multiple extracranial metastases. We believe that it would probably be worthwhile to investigate predictive models and nomograms which might be able to predict BM in asymptomatic patients, when the disease burden is low and there is effective local treatment available possibly in the form of SRS or resection.
References
1. Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol 2005;75:5–14.
2. Nichols EM, Patchell RA, Regine WF, Kwok Y. Palliation of brain and spinal cord metastases. In: Halperin EC, Wazer DE, Perez CA, Brady LW, editors. Principles and practice of radiation oncology. 6th edition. Philadelphia: Lippincott Williams &Wilkins; 2013. p. 1766-78.
3. Pérez-Larraya JG, Hildebrand J. Brain metastases. In: Biller J, Ferro JM. editors. Handbook of Clinical Neurology. Vol. 121. Amsterdam: Elsevier BV; 2014.p.1143-57.
4. Schellinger P.D, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CT in patients with brain metastases. J Neurooncol. 1999; 44: 275-81.
5. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002; 94 : 2698-2705.
6. Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. B J cancer 2009;101:1919–24.
7. Tsakonas G, Hellman F, Gubanski M, Friesland S , Tendler S, Lewensohn R ,et al. Prognostic factors affecting survival after whole brain radiotherapy in patients with brain metastasized lung cancer. Acta Oncol 2018 Feb;57(2):231-8.
8. Hochstenbag MM, Twijnstra A, Wilmink JT, Wouters EF, Velde GP. Asymptomatic brain metastases (BM) in small cell lung cancer (SCLC): MR-imaging is useful at initial diagnosis. J Neurooncol 2000; 48 : 243-8.
9. Patel AJ, Lang FF, Suki D, Wildrick D.M, Sawaya R. Metastatic brain tumors. In: Winn HR, editors. Youmans and Winn Neurological Surgery. 7th edition. Philadelphia: Elsevier PA; 2017.chapter No. 146.
10. David PC ,Hudgins PA ,Peterman SB, Hoffman JC . Diagnosis of cerebral metastasis: double dose delayed CT vs contrast enhanced MR imaging .Am J Neuroradiol 1991;12:293-300 .
11. Murray KJ, Scott C, Greenberg HM, Emami B, Seider M, Vora NL, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: A report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys 1997; 39: 571- 4.
12. Langer CJ and Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23: 6207-19.
13. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1–9.
14. Zimm S, Wampler G.L, Stablein D , Hazra T, Young HF . Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer 1981;48:384–94.
15. Egawa S, Tukiyama I, Akine Y, Kajiura Y,Yanagawa S,Watai K, et al. Radiotherapy of brain metastases. Int J Radiat Oncol Biol Phys 1986;12:1621–5.
16. Pladdet I, Boven E, Nauta J, Pinedo H.M. Palliative care for brain metastases of solid tumour types. Neth J Med 1989;34:10–21.
17. Komarnicky LT, Phillips TL, Martz K, Asbell S, Isaacson S, Urtasun R. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys 1991;20:53–8.
18. Nieder C, Niewald M, Schnabel K. The radiotherapy of brain metastases in bronchial carcinoma. StrahlentherOnkol 1994;170:335–41
19. Ryan GF, Ball DL, Smith JG. Treatment of brain metastases from primary lung cancer. Int J Radiat Oncol Biol Phys 1995;31:273–8.
20. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997; 37: 745–51.
21. Lagerwaard FJ, Levendag PC. Prognostic factors in patients with brain metastases. Forum (Genova) 2001;11:27-46.
22. Hazuka MB, Burleson WD, Stroud DN, Leonard CE, Lillehei KO, Kinzie JJ, et al. Multiple brain metastases are associated with poor survival in patients treated with surgery and radiotherapy. J Clin Oncol 1993;11:369-73.
23. Lentzsch S, Reichardt P, Weber F, Budach V, Dörken B. Brain metastases in breast cancer: Prognostic factors and management. Eur J Cancer 1999;35:580-5.
24. Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999; 43:795–803.
25. Weltman E, Salvajoli JV, Brandt RA, de MoraisHanriot R, Prisco FE, Cruz JC, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 2000; 46:1155–61.
26. Lorenzoni J, Devriendt D, Massager N, David P, Ruiz S, Vanderlinden B, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys 2004; 60:218–24.
27. Golden DW, Lamborn KR, McDermott W, Kunwar S, Wara WM, Nakamura JL, et al. Prognostic factors and grading systems for overall survival in patients treated with radiosurgery for brain metastases: variation by primary site. J Neurosurg 2008; 109 Suppl :77–86.
28. Rades D, Dunst J, Schild SE. A new scoring system to predicting the survival of patients treated with whole-brain radiotherapy for brain metastases. Strahlentherapie und Onkologie: Organ der Deutschen Rontgen gesellschaft 2008 ;184:251–5.
29. Rades D, Dziggel L, Haatanen T, Veninga T, Lohynska R, Dunst J, et al. Scoring systems to estimate intracerebral control and survival rates of patients irradiated for brain metastases. Int J Radiat Oncol Biol Phys 2011; 80:1122–7.
30. Sperduto W, Kased N, Roberge Z, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2011; 30(4): 419-25.
31. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi- institutional analysis of 4259 patients. Int J Radiat Oncol Biol Phys 2010; 77(3): 655-61.
32. Mulvenna P, Nankivell M, Barton R, Faivre-Finn C,Wilson P, NcColl E, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomized trial. Lancet 2016;388:2004–14.
33. Saha A, Ghosh SK, Roy C, Choudhury KB, Chakrabarty B, Sarkar R, et al. Demographic and clinical profile of patients with brain metastases: A retrospective study. Asian J Neurosurg 2013;8:157-61.
34. Ekici K, Temelli O, Dikilitas M, HalilDursun I, Bozdag Kaplan N, Kekilli E, et al. Survival and prognostic factors in patients with brain metastasis: Single center experience. J BUON 2016;21:958- 63.
35. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE . Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865.
36. Walker A.E, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology 1985;35:219.
37. Suki D. The epidemiology of brain metastasis. In: Sawaya R, editor. Intracranial metastases: current management strategies. Malden (MA): Blackwell; 2004. p. 20.
38. Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W,et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol 2006;13:674-81.
39. Akhavan A, Binesh F, Heidari S. Survival of brain metastatic patients in Yazd, Iran. Asian Pac J Cancer Prev 2014;15:3571-4.
40. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors, Neurosurg Clin N Am 2011; 22 :1(1-6).
41. Mujoomdar A, Austin JHM, Malhotra R, Powell CA, Pearson GDN, Shiau MC, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 2007;242:882–8.
42. Nieder C, Marienhagen K, Geinitz H, Molls M. Validation of the graded prognostic assessment index for patients with brain metastases. Acta Oncol 2009; 48: 457-9.
43. Nieder C, Spanne O, Mehta MP, Grosu A.L, Geinitz H. Presentation, patterns of care and survival in patients with brain metastases: what has changed in the last 20 years? Cancer 2011;117(11):2505– 12.
44. Nieder y K, Marienhagen ZA, Dalhaug G, Aandahl E, Haukland AP Prognostic Models Predicting Survival of Patients with Brain Metastases: Integration of Lactate Dehydrogenase, Albumin and Extracranial Organ Involvement . J Clinc Oncol 2014;26 : 447-52.
45. Lewitzki V, Klement RJ, Hess S, Kosmala R, Nieder C, Flentje M. External validation of a prognostic score predicting overall survival for patients with brain metastases based on extracranial factors. Clin Transl Radiat Oncol 2019 May; 16: 15–20.
46. Zhang X, Zhang W, Cao WD, Cheng G, Liu B, Cheng J. A review of current management of brain metastases. Ann Surg Oncol 2012;19(3):1043–50.
47. Soffietti R, Ruda R, Mutani R. Management of brain metastases. J Neurol 2002; 249(10):1357–69.
48. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48–54.
49. Benna1 M, Mejri N, Mabrouk M, El Benna H, Labidi S, Daoud N. Brain metastases epidemiology in a Tunisian population: trends and outcome. CNS Oncol 2018 ;7(1):35–39.
50. Delattre J.Y, Krol G, Thaler HT ,Posner B. Distribution of brain metastases. Arch Neurol 1988;45:741–4.
51. Ozgül M.A, Uysal MA, Kadakal F, Altoparlak B, Cinemre H, Yilmaz V. Comparison of computed tomography and magnetic resonance imaging to diagnosis of Brain metastasis in non-small cell lung cancer. Tuberkuloz ve toraks 2006; 54(3):229-34.
52. Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol 1980;7 (6)
: 529–41.
53. Hjorthaug K, Hojbjerg J, Knap M, Tietze A, Haraldsen A, Zacho HD, et al. Accuracy of 18F-FDG PET/CT in triaging lunch cancer patients with suspected brain metastases for MRI. Nucl Med Commun 2015;36:1084–90.
54. Sheehan J, Kondziolka D, Flickinger J , Lunsford LD. Radiosurgery for patients with recurrent small cell lung carcinoma metastatic to the brain: Outcomes and prognostic factors. J Neurosurg 2005; 102(Suppl): 247-54.
55. Sheehan JP, Sun MH, Kondziolka D, Flickinger J , Lunsford LD. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: Long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 2002; 97: 1276-81.
56. Le SR, Massard C, Mouret-Fourme E, Guinebretierre JM, CohenSolal C, De L.B., et al. Brain metastases from breast carcinoma: Validation of the radiation therapy oncology group recursive partitioning analysis classification and proposition of a new prognostic score. Int J Radiat Oncol Biol Phys 2007; 69: 839-45 .
57. Niwinska A, Murawska M , Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer 2010 ; 116: 4238-47.
58. Niwinska A, Murawska M , Pogoda K. Breast cancer brain metastases: Differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Ann Oncol 2010; 21: 942-8.
59 .Wong E, Tsao M, Chow E. Survival of patients with multiple brain metastases treated with whole- brain radiotherapy. CNS Oncol 2015 Jul;4(4):213-24.
60. Priestman TJ, Dunn J, Brada M, Rampling R, Baker PG. Final results of the Royal College of Radiologists’ trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin. Oncol. (R. Coll. radiol.) 1996;8(5):308–15.
61. Fleckenstein K, Hof H, Lohr F, Wenz F, Wannenmacher M . Prognostic factors for brain metastases after whole brain radiotherapy. Data from a single institution. Strahlenther Onko 2004;180(5):268–73.
62. Silva MG, Gomes FC, Saito EY, Maia MAC, Salvajoli JV. Whole-brain radiotherapy in the treatment of brain metastases: a 12 year analysis of prognostic factors and survival. Int J Rad Onc Biol Phys 2010;78(3):S584–S85.
63. Chan S, Rowbottom L, McDonald R, Zhang L , Bjarnason GA, Tsao M, et al. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann Palliat Med 2016;5(4):267-79.
64. Jakhar S, Kapoo A, Singh D, Patidar AK, Hirapara PH, Kumar HS. Prognostic factors affecting the survival of patients with brain metastasis treated by whole brain radiotherapy: A regional cancer center experience from North West India. Clin Cancer Res 2015; 4 ( 1) : 29-33.
65. Jeene PM, de Vries KC, van Nes JGH , Kwakman JJM , Wester G, Rozema T, et al. Survival after whole brain radiotherapy for brain metastases from lung cancer and breast cancer is poor in 6325 Dutch patients treated between 2000 and 2014. Acta Oncologica 2018 ; 57(5): 637–43.
66. Thakur P, Sharma A, Gupta M, Dhiman A, Sharmaet J. Prognostic Factors and Survival Outcome of Whole Brain Radiotherapy in Metastatic Brain Cancer- A Single Regional Cancer Centre Experience in North India .J Evolution Med Dent 2019 Oct ;8: 3206-11.
67. Sundstrom JT, Minn H, Lertola KK, Nordman E . Prognosis of patients treated for intracranial metastases with whole brain irradiation. Ann Med 1998;30(3):296-9.
68. Miyazawa K, Shikama N, Okazaki S, Koyama T, Takahashi T, Kato S. Predicting prognosis of short survival time after palliative whole-brain radiotherapy .J Rad Res2018 Jan; 59(1): 43–49.
69. Pesce G.A, Klingbiel D, Ribi K, Zouhair A, Von MR, Schlaeppi M, et al. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole-brain radiotherapy combined with gefitinib or temozolomide. A randomized phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer 2011; 48: 377-84.
70. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients With Brain Metastases. J Clin Oncol 2012 Feb 1; 30(4): 419–25.
71. Wang M, Wu Q, Zhang J, Qin G, Yang T, Liu Y,et al. Prognostic impacts of extracranial metastasis on non-small cell lung cancer with brain metastasis: A retrospective study based on surveillance, epidemiology, and end results database.Cancer Medicine 2021 June ; 10 (12) :471-82.
72. Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, Schild S.E, et al. Brain metastasis from non- small cell lung cancer (NSCLC). Strahlenther Onkol 2014;190:64–67.
73. Gerdan L, Šegedin B, Veninga T , Schild SE, Rades D. Number of Involved Extracranial Organs Predicts Survival in Patients with Brain Metastasis from Small Cell Lung Cancer. Anticancer Res 2013; 33: 3887-90 .
74. Gerdan L, Segedin B, Nagy V, Khoa MT, Trang NT, Schild SE,et al . The number of involved extracranial organs: a new predictor of survival in breast cancer patients with brain metastasis. Clin Neurol Neurosurg 2013; 115: 2108– 10.
75. Noordijk EM, Vecht CJ, Haaxma-Reiche H, Padberg GW, Voormolen JH, Hoekstra FH, et al. The choice of treatment of single brain metastasis should be based on extra-cranial activity and age. Int J Radiat Oncol Biol Phys 1994; 29:711-17.
76. PhillipsTL, Scott CB, Leibel S.A, Rotman M, Weigensberg IJ. Results of a randomized comparison of radiotherapy and bromodeoxyurine to radiotherapy alone for brain metastases: Report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys 1994; 30:215.
77. Sen M, Demiral AS, Cetingöz R, Alanyali H, Akman F, Sentürk D, et al. Prognostic factors in lung cancer with brain metastasis. Radiother Oncol 1998;46:33-8.
78. Sánchez de Cos J, Sojo González M A, Montero MV, Pérez Calvo MC, Vicente MJM, Valle MH. Non- small cell lung cancer and silent brain metastasis: Survival and prognostic factors. Lung Cancer 2009; 63: 140–5.
79. Ampil F, Caldito G, Milligan S, Mills G, Nanda A. The elderly with synchronous non-small cell lung cancer and solitary brain metastasis: Does palliative thoracic radiotherapy have a useful role? .Lung Cancer 2007; 57: 60–5.
80. Penel N, Brichet A, Prevost B , Duhamel A , Assaker R, Dubois F, Lafitte JJ . Pronostic factors of synchronous brain metastases from lung cancer. Lung Cancer 2001; 33: 143–54.
81. Mehta MP, Shapiro WR, Phan .C, Gervais R, Carrie C, Chabot P,et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small- cell lung cancer patients with brain metastases: results of a phase III trial. Inter Radiat Oncol Biol Phy 2009; 73(4):1069–76.
82. Hansen C, Janssen S, Cathrin Thieme C, Alekse J , Shield S E. Potential Impact of the Interval Between Imaging and Whole-brain Radiotherapy in Patients With Relatively Favorable Survival Prognoses. Anticancer Res 2019; 39 (3) :1343-46.
83. Nieder ., Norum J, Dalhaug A, Aandahl G, Engljähringer K. Best supportive care in patients with brain metastases and adverse prognostic factors: development of improved decision aids. Support Care Cancer 2013; 21: 2671-8.
84. Rades D, Bohlen G, Dunst J, Lohynska R, Veninga T, Stalpers L, et al. Comparison of short-course versus long-course whole-brain radiotherapy in the treatment of brain metastases. Strahlenther Onkol 2008;184: 30-5.
85. Harada H, Asakura H, Ogawa H, Mori K, Takahashi T, Nakasu Y,et al. Prognostic factors in patients with brain metastasis from non-small cell lung cancer treated with whole-brain radiotherapy. J Can Res Ther 2016;12:267–70.
86. Broadbent AM, Hruby G, Tin M.M, Jackson M, Firth I. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother. Oncol 2004;71(3):259–65.
87. Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol 2000;17(4):279–86.
88. Staudt M, Lasithiotakis K, Leiter U, F Meier, Eigentler T, Bamberg M, et al . Determinants of survival in patients with brain metastases from cutaneous melanoma. British J Cancer 2010; 102: 1213–18 .
89. Patil R , Pandit P , Palwe V , Kate S , Gandhe S , Patil R, et al. Evaluation of Prognostic Factors that Affect Survival Outcomes of Breast Cancer Patients with Brain Metastases: A Single Institutional Experience. Eur J Breast Health 2021; 17(1): 62-7.
90. Saito EY, Viani GA, Ferrigno R, Nakamura RA, Novaes PE, Pellizzon CA,et al .Whole brain radiation therapy in management of brain metastasis: results and prognostic factors. Rad Oncol 2006; 1(20):1-7.
91. Sperduto P.W, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 2008; 70:510–4.
92. Fabi A, Felici A, Metro G, Mirri A, Bria E, Telera S, et al. Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res 2011;30:10.
93. Waqar SN, Samson PP, Robinson CG, Bradley J, Devarakonda S. Non-small cell lung cancer with brain metastasis at presentation. Clin Lung Cancer 2018 Jul; 19(4): e373–e379.
94. Zhang J, Xu J, Jin S, Gao W, Guo R, Chen L. The development and validation of a nomogram for predicting brain metastases in lung squamous cell carcinoma patients: an analysis of the Surveillance, Epidemiology, and End Results (SEER) database. J thorac Dis 2021;13(1):270-81.
95. Yokoi K, Miyazawa N, Arai T. Brain Metastasis in Resected Lung Cancer: Value of Intensive Follow-up With Computed Tomography. Ann Thorac Surg 1996;61:546-51.

Figure 1
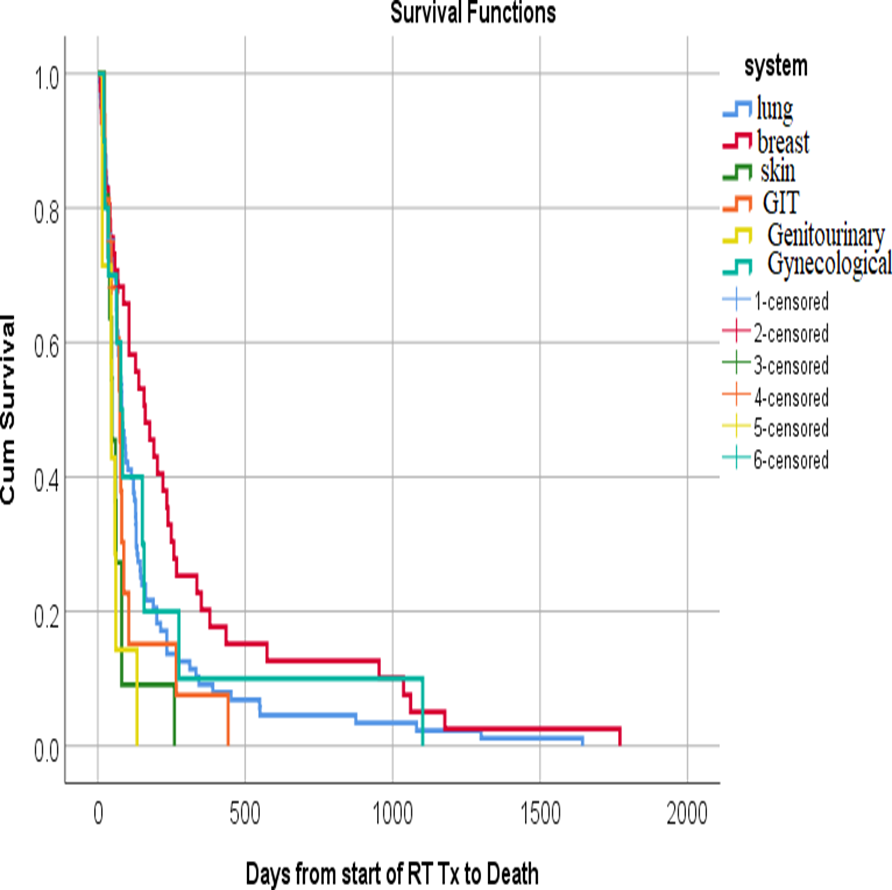
Figure 2
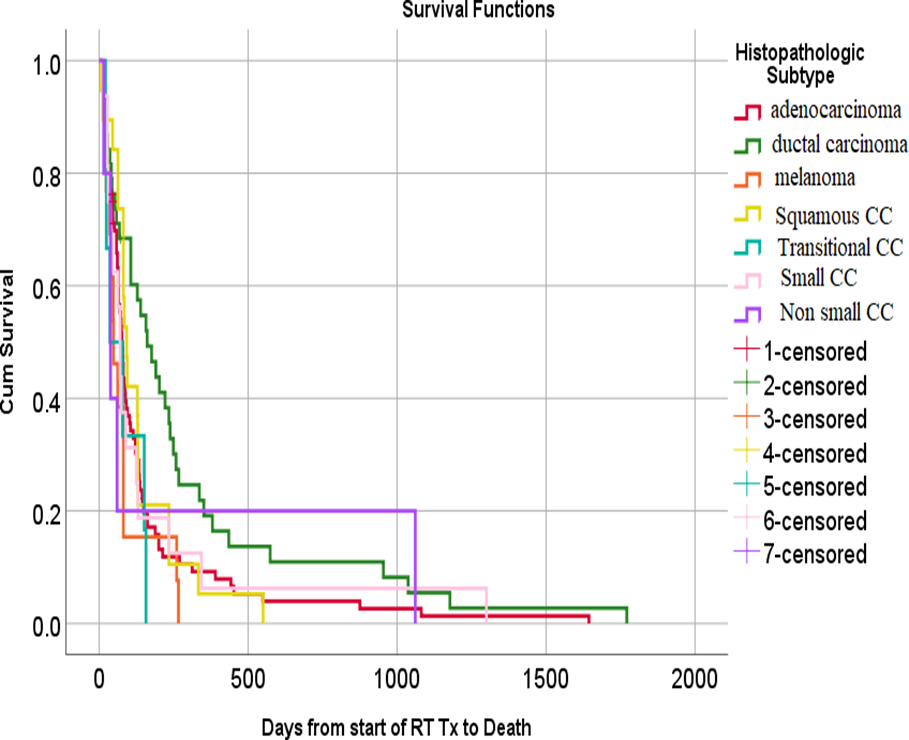
Figure 3
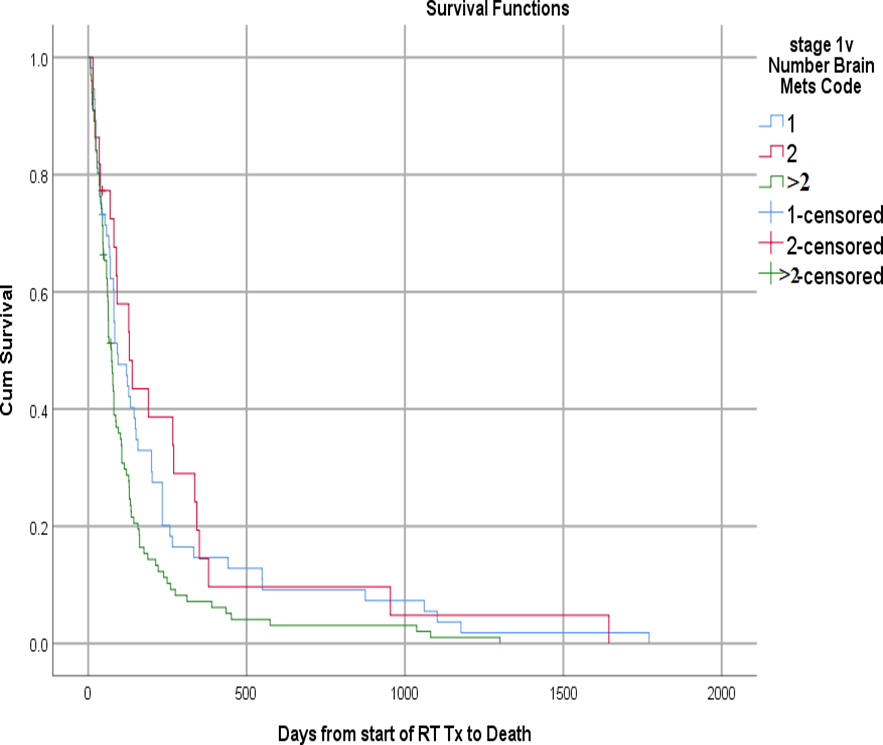
Figure 4
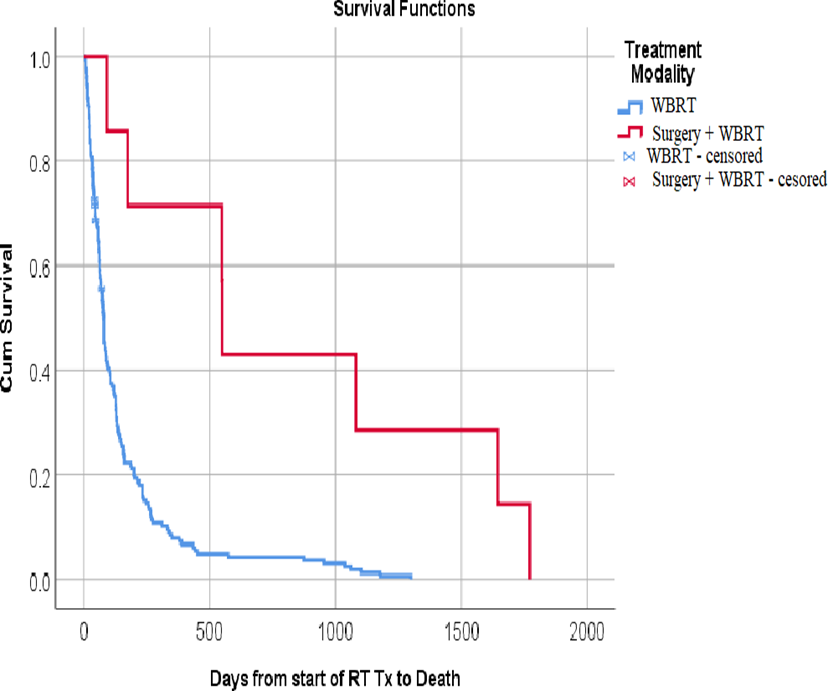
Figure 5
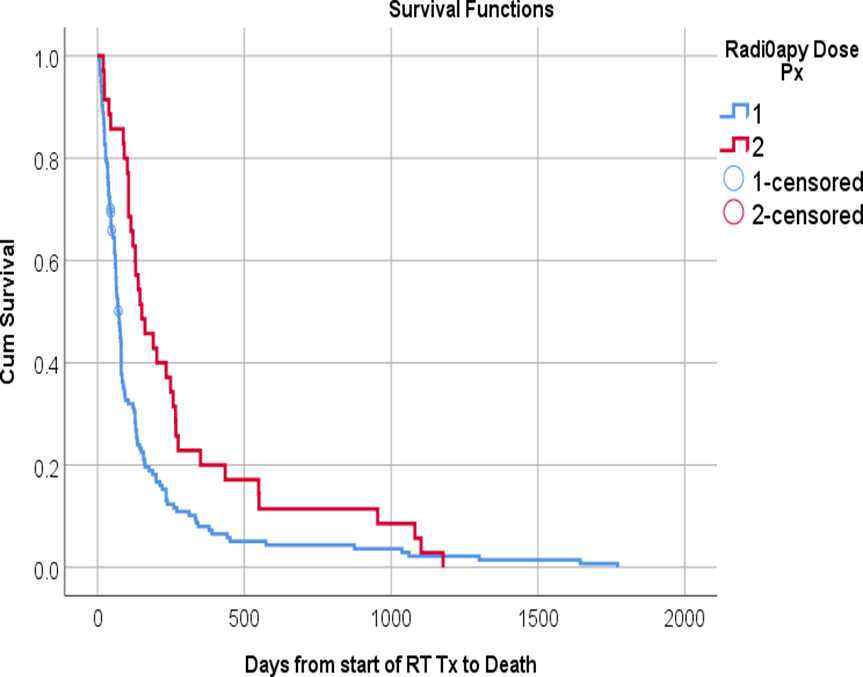
Figure 6
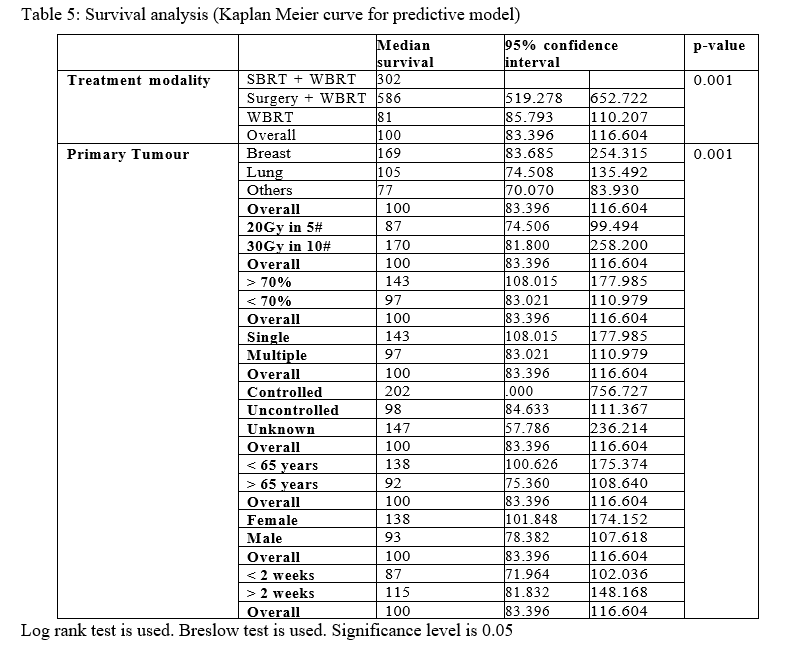
Figure 7
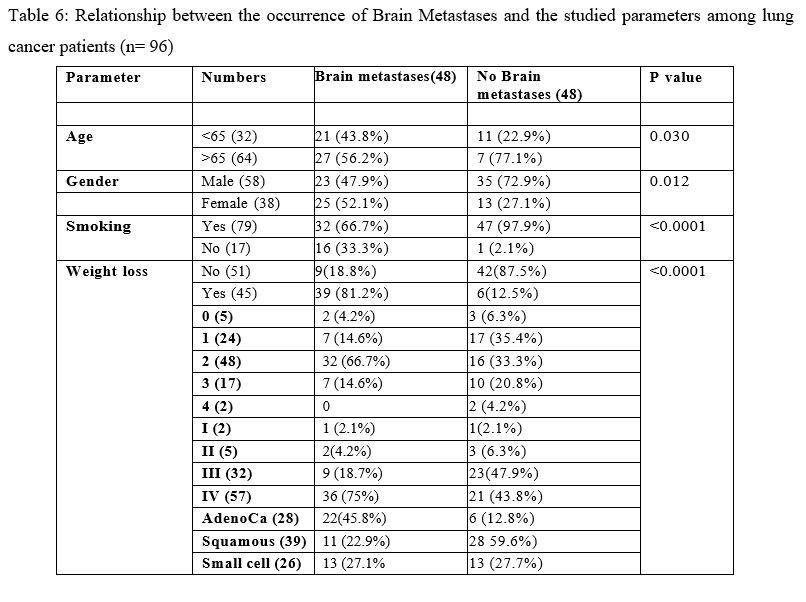
Figure 8
