Refeeding Syndrome – Underestimated Clinical Problem in Everyday Practice
Refeeding Syndrome – Underestimated Clinical Problem in Everyday Practice
Vinod Kumar Singhal 1*, Adil Mohammed Suleman2, Nufra Senopher3, Varsha Ojha4, Archana Singh5
1. Vinod Kumar Singhal, Consultant surgeon, Department of General Surgery, Prime hospital, Dubai, UAE
2.Adil Mohammed Suleman, Specialist General Surgeon, Department of General Surgery, Prime Hospital, Dubai, UAE.
3. Nufra Senopher, Department of General Surgery, Prime Hospital, Dubai, UAE.
4. Varsha Ojha- Specialist Gynecologist and Obstetrician, Prime Hospital, Dubai, UAE
5.Archana Singh- IVF specialist, Gynecologist & Obstetrician, Indra IVF, Jodhpur, India
*Correspondence to Vinod Kumar Singhal, Consultant surgeon, Department of General Surgery, Prime hospital, Dubai, UAE.
Copyright
© 2024 Vinod Kumar Singhal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 20 September 2024
Published: 23 September 2024
Refeeding Syndrome – Underestimated Clinical Problem in Everyday Practice
Introduction
Refeeding syndrome (RFS) is a series of metabolic and electrolyte changes that occur as a result of reintroduction and/or increased calorie delivery after a period of reduced intake [1]. It manifests as acute electrolyte deficiency with associated fluid retention and glucose disturbances as a result of oral, enteral or parenteral nutrition in malnourished patients [2].
Despite the fact that the first reports on the symptoms of RFS date back to the 5th century BC and were confirmed and detailed in the 1940s, there is still insufficient awareness of it among doctors and nurses. Considering that 30-55% of patients admitted to hospitals are malnourished at the time of admission, the appropriate implementation of nutrition and the treatment of possible symptoms of the Refeeding Syndrome is clinically important[3, 4].
The first cases of Refeeding Syndrome described in detail date back to the end of the Second World War, when prisoners of war released from concentration camps, starved for months, began to take their meals. Many of them died within days of liberation [5]. Following these observations, Keys conducted an experiment in which he studied the physical and psychological effects of long-term dietary restriction and subsequent re-feeding in 36 study participants. The majority of subjects experienced severe emotional distress, depressive symptoms, social withdrawal, isolation, decreased concentration and decreased metabolic rate, slowed respiration rate and heart rate, swelling of the extremities during the periods [6]. Further observations were made by Schnitker and Burger. A number of starved inmates developed severe symptoms such as heart failure, peripheral oedema and neurological disorders after a normal diet was restored, and one inmate died within the next few days [7]. These observations led to the first description of Refeeding Syndrome (RFS).
Pathogenesis of RFS
RFS is an intense physiological response to glucose reintroduction (re-feeding) after a prolonged phase of starvation or restricted food intake[8]. The exact pathophysiological mechanisms remain unclear, but assumptions are based on the processes shown in Fig.1.
Fig. 1.Pathophysiology of the Refeeding Syndrome (adapted from Stanga Z. et el.) [9]
In a catabolic state (due to reduced food intake or starvation), insulin production is reduced, glucose oxidation takes place only in glucose-dependent tissues such as the brain, renal medulla and red blood cells. Glycogen stores are reduced, leading to the activation of gluconeogenesis and the production of glucose from endogenous amino acids, which are released by increased proteolysis. This process results in reduced muscle mass, causing functional weakness and weight loss. Vitamin and electrolyte levels are reduced and their stores are depleted. After a few days, lipolysis increases, leading to increased levels of free fatty acids in the circulation. Free fatty acids stimulate ketogenesis in the liver, leading to a high production of ketone bodies, which become the body's main energy providers. If refeeding begins, glucose becomes the main energy supplier, causing hyperglycaemia and consequently an increase in insulin secretion. Anabolic processes are stimulated, leading to intracellular shifts of glucose, water and electrolytes, resulting in a potentially severe drop in serum micronutrient levels. The resulting electrolyte imbalance can cause life-threatening complications such as arrhythmia, cramps or tetany. At the same time, thiamine deficiency can lead to neurological and cardiorespiratory symptoms of beri-beri disease. The more impaired the nutritional status of the patient , the greater the risk of RFS and the greater the severity of its symptoms[1, 10, 11].
Thiamine is an essential cofactor in carbohydrate metabolic pathways, enabling the conversion of glucose to adenosine triphosphate (ATP) in the Krebs cycle. In thiamine deficiency, glucose is converted to lactate, leading to metabolic acidosis. Thiamine deficiency can also cause neurological (Wernicke's encephalopathy) or cardiovascular disorders (the so-called 'wet' form of beri-beri disease)[12, 13]
Clinical consequences resulting from electrolyte changes and thiamine deficiency may include.[14-16]:
• tachycardia, tachypnoea, oedema
• hyper/hypotension, cardiomyopathy, sudden cardiac arrest
• pulmonary oedema
• paresthesias, convulsions
• constipation, abdominal pain, diarrhoea
• metabolic acidosis
The relationships between electrolyte deficiencies and symptoms are shown in Fig.2.
Fig.2. Electrolyte disturbances and symptoms associated with Refeeding Syndrome [11, 17-19]
|
Hypophosphatemia |
nausea, vomiting, heart failure, arrhythmias, haemolytic anaemia, pancytopenia, rhabdomyolysis, acute tubular necrosis, cranial nerve palsy, muscle paralysis, disorientation, coma. |
|
Hypomagnesaemia |
Hypocalcaemia, cardiac arrhythmias, tachycardia, tremor, ataxia, confusion, irritability, paresthesias, abdominal pain, convulsions, tetany. |
|
Hypokalaemia |
Arrhythmia, hyporeflexia, low blood pressure, paralytic bowel obstruction, paresthesias, spasms, muscle paralysis, respiratory depression, myoglobinuria, polyuria, metabolic alkalosis. |
|
Thiamine deficiency |
Wernicke's encephalopathy, syndrome Korsakoff |
|
Carbohydrate intolerance, fluid intolerance |
hyperosmolarity, hepatic steatosis. Dehydration, peripheral oedema, heart failure, low blood pressure, pre-renal renal failure, sudden death |
Prevention of Relapse Syndrome
In order to prevent the onset of RFS symptoms, risk factors need to be kept in mind and assessed for each patient in whom enteral nutrition, parenteral nutrition or extended oral diet is initiated [16]. Risk factors for the relapse syndrome are shown in Fig.3.
Fig.3. Risk factors for Relapse Syndrome [10]
It is worth noting that patients at high risk of Refeeding Syndrome are also patients at high nutritional risk according to the NRS 2002 scale. This scale is compulsorily completed in every hospitalised patient (Fig.4). A score equal to or higher than 3 points requires the inclusion of nutritional treatment. In this situation, RFS prevention should be kept in mind.
Fig.4. NRS 2002 scale
|
Deterioration of nutritional status |
Severity of illness (increased demand on nutrients) |
||
|
0 = none |
correct nutritional status |
0 = none |
normal demand |
|
1 = mild malnutrition |
weight loss >5% during 3 months Or food intake <50-75% in the past week |
1 = mild malnutrition |
hip fracture, chronic diseases especially with complications - cirrhosis, COPD, diabetes, cancer |
|
2 = medium malnutrition |
weight loss >5% during 2 months or BMI 18.5-20.5 kg/m2 with associated poor general condition or a food intake in the range of 25-60% of normal requirements in the last week |
2 = medium malnutrition |
major abdominal procedures, stroke, severe pneumonia, post-operative renal failure, chemotherapy, malignant haematological diseases |
|
3 = severe malnutrition |
weight loss >5% during 1 month (>15% over 3 months) or BMI < 18.5 kg/m 2 with associated poor general condition or food intake between 0-25% of normal requirements in the last week |
3 = severe malnutrition |
head injury, Bone marrow transplant, patients requiring intensive medical therapy |
|
1 = if patient's age >70 years |
|||
|
Total number of points = |
|||
When starting to feed a patient, risk factors should be taken into account and the amount of kcal, composition and feeding rate should be adjusted accordingly (Figure 5).
Fig.5 Prevention of RFS in hospitalized patients receiving nutritional treatment [10]
RFS prevention
- identification of patients at risk of RFS
- assessment of electrolyte concentrations and correction of deficiencies before starting feeding
- administration of thiamine before feeding
- selecting an appropriate supply: 10-15kcal/kg/24h for the first 3 days of nutritional treatment
- evaluation of symptoms and test results following the inclusion of nutrition
Recognition of RFS
RFS can occur in up to 62% of hospitalized patients, depending on their nutritional status and general condition [20].
In 2017. ASPEN (American Society for Parenteral and Enteral Nutrition) proposed the following diagnostic criteria for RSF:
Decrease in serum phosphorus, potassium and/or magnesium (occurring within 5 days of reintroduction of nutrition):
• by 10%-20% (mild form)
• by 20%-30% (moderate form)
• by >30% and/or organ dysfunction due to reduction in any of the organs and/or due to thiamine deficiency (severe) [1, 21, 22]
RFS treatment
Currently, there are no standardized, evidence-based guidelines for the treatment of RFS. However, it is assumed, based on previous studies, that an effective risk assessment strategy, development of a nutritional care plan and monitoring of patients at risk of RFS can significantly reduce the morbidity and mortality associated with this syndrome [10, 23-25].
If symptoms of the Refeeding Syndrome are already present, nutritional support should be reduced and hypophosphatemia, hypokalaemia and hypomagnesaemia should be corrected immediately, while treating the patient's other health problems [26, 27]. Moderately or severely ill patients with seizures, significant oedema or serum phosphorus levels <2 mg/dl should be hospitalized for intravenous electrolyte supplementation and close monitoring.
In summary, when symptoms of RFS occur, it is important to [16, 23, 28]:
1. stop the supply of nutrition
2. assess electrolyte concentrations
3. replenish electrolyte deficiencies
4. administer thiamine
5. reintroduce feeding at the correct dose and rate
Summary
Refeeding Syndrome is a potentially fatal metabolic complication of nutritional treatment.
Correct assessment of need and estimation of RFS risk are key to the correct inclusion of oral, enteral and/or parenteral nutrition.
Prompt recognition of RFS symptoms and appropriate diagnostic and therapeutic management makes it possible to reduce or resolve symptoms.
References
1. da Silva, J.S.V., et al., ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr Clin Pract, 2020. 35(2): p. 178-195.
2. Adika, E., et al., Evaluation of the ASPEN guidelines for refeeding syndrome among hospitalized patients receiving enteral nutrition: A retrospective cohort study. JPEN J Parenter Enteral Nutr, 2022. 46(8): p. 1859-1866.
3. Stephenson, S.S., et al, The Relationship between Nutritional Risk and the Most Common Chronic Diseases in Hospitalized Geriatric Population from Central Poland. Nutrients, 2023. 15(7).
4. Friedli, N., et al., Revisiting the refeeding syndrome: Results of a systematic review. Nutrition, 2017. 35: p. 151-160.
5. Solomon, S.M. and D.F. Kirby, The refeeding syndrome: a review. JPEN J Parenter Enteral Nutr, 1990. 14(1): p. 90-7.
6. LASKER, G.W., The effects of partial starvation on somatotype: an analysis of material from the Minnesota starvation experiment. Am J Phys Anthropol, 1947. 5(3): p. 323-41.
7. SCHNITKER, M.A., A study of malnutrition in Japanese prisoners of war. J Mil Med Pac, 1946. 2(3): p. 3-19.
8. Rinninella, E., et al., Incidence and Impact of Refeeding Syndrome in an Internal Medicine and Gastroenterology Ward of an Italian Tertiary Referral Center: A Prospective Cohort Study. Nutrients, 2022. 14(7).
9. Stanga, Z., et al., Nutrition in clinical practice-the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr, 2008. 62(6): p. 687-94.
10. Friedli, N., et al., Management and prevention of refeeding syndrome in medical inpatients: An evidence-based and consensus-supported algorithm. Nutrition, 2018. 47: p. 13-20.
11. Wong, G.J.Y., et al., Refeeding Hypophosphatemia in Patients Receiving Parenteral Nutrition: Prevalence, Risk Factors, and Predicting Its Occurrence. Nutr Clin Pract, 2021. 36(3): p. 679-688.
12. Mehanna, H.M., J. Moledina, and J. Travis, Refeeding syndrome: what it is, and how to prevent and treat it. BMJ, 2008. 336(7659): p. 1495-8.
13. Brooks, M.J. and G. Melnik, The refeeding syndrome: an approach to understanding its complications and preventing its occurrence. Pharmacotherapy, 1995. 15(6): p. 713-26.
14. Naik, N.M., et al., Assessment of refeeding syndrome definitions and 30-day mortality in critically ill adults: A comparison study. JPEN J Parenter Enteral Nutr, 2023. 47(8): p. 993-1002.
15. Pantoja, F., et al., Refeeding syndrome in adults receiving total parenteral nutrition: An audit of practice at a tertiary UK centre. Clin Nutr, 2019. 38(3): p. 1457-1463.
16. Reber, E., et al, Management of Refeeding Syndrome in Medical Inpatients. J Clin Med, 2019. 8(12).
17. Buitendag, J., et al., Refeeding syndrome in surgical patients post initiation of artificial feeding, a prospective cohort study in a low-income country. Clin Nutr ESPEN, 2021. 46: p. 210-215.
18. Jeon, T.J., et al., Refeeding Syndrome as a Possible Cause of Very Early Mortality in Acute Pancreatitis. Gut Liver, 2019. 13(5): p. 576-581.
19. Liu, P., et al., Impact of calorie intake and refeeding syndrome on the length of hospital stay of patients with malnutrition: a systematic review and meta-analysis. Clin Nutr, 2022. 41(9): p. 2003-2012.
20. Cioffi, I., et al., The incidence of the refeeding syndrome. A systematic review and meta-analyses of literature. Clin Nutr, 2021. 40(6): p. 3688-3701.
21. Statlender, L., et al., Correlations between First 72 h Hypophosphatemia, Energy Deficit, Length of Ventilation, and Mortality-A Retrospective Cohort Study. Nutrients, 2022. 14(7).
22. Xiong, R., et al., Incidence and outcome of refeeding syndrome in neurocritically ill patients. Clin Nutr, 2021. 40(3): p. 1071-1076.
23. Krutkyte, G., et al., Refeeding Syndrome: A Critical Reality in Patients with Chronic Disease. Nutrients, 2022. 14(14).
24. Nutrition support in adults: Evidence Update August 2013: A summary of selected new evidence relevant to NICE clinical guideline 32 'Nutrition support in adults: oral nutrition support, enteral tube feeding and parenteral nutrition' (2006). 2013.
25. Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. 2017.
26. Fuentebella, J. and J.A. Kerner, Refeeding syndrome. Pediatr Clin North Am, 2009. 56(5): p. 1201-10.
27. Mehler, P.S., et al., Nutritional rehabilitation: practical guidelines for refeeding the anorectic patient. J Nutr Metab, 2010. 2010.
28. Terlisten, K., et al., Refeeding Syndrome in Older Hospitalized Patients: Incidence, Management, and Outcomes. Nutrients, 2023. 15(18).
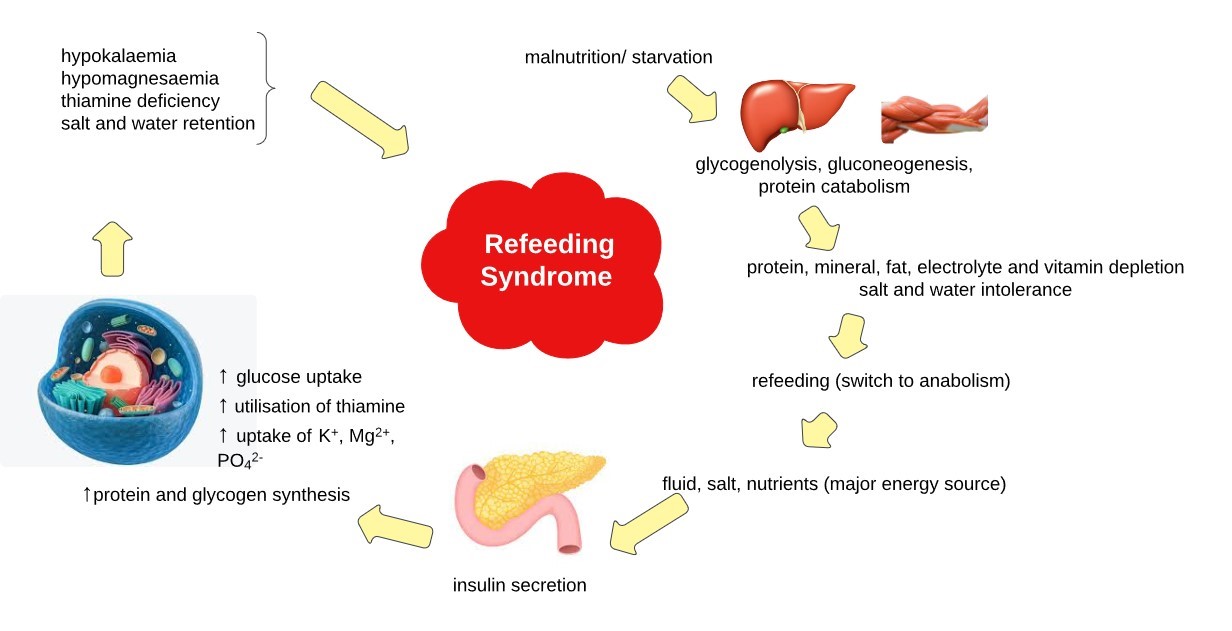
Figure 1
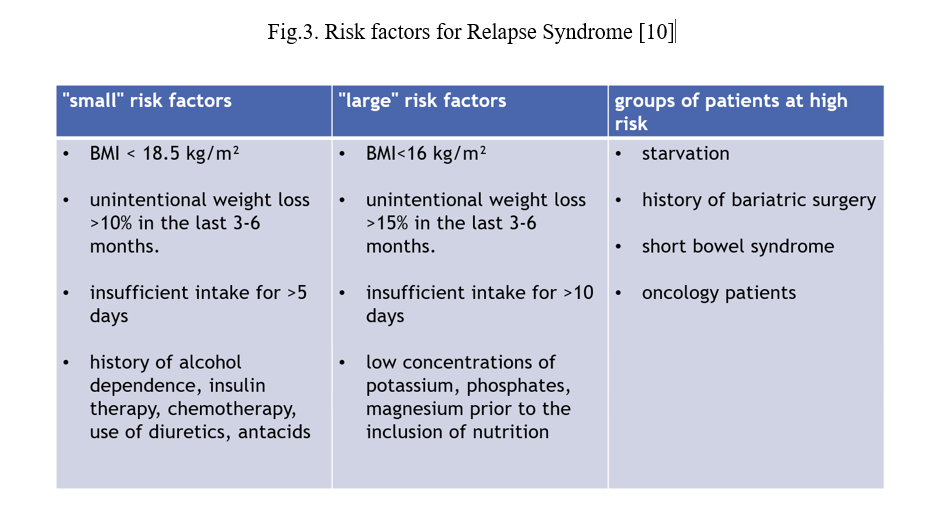
Figure 2
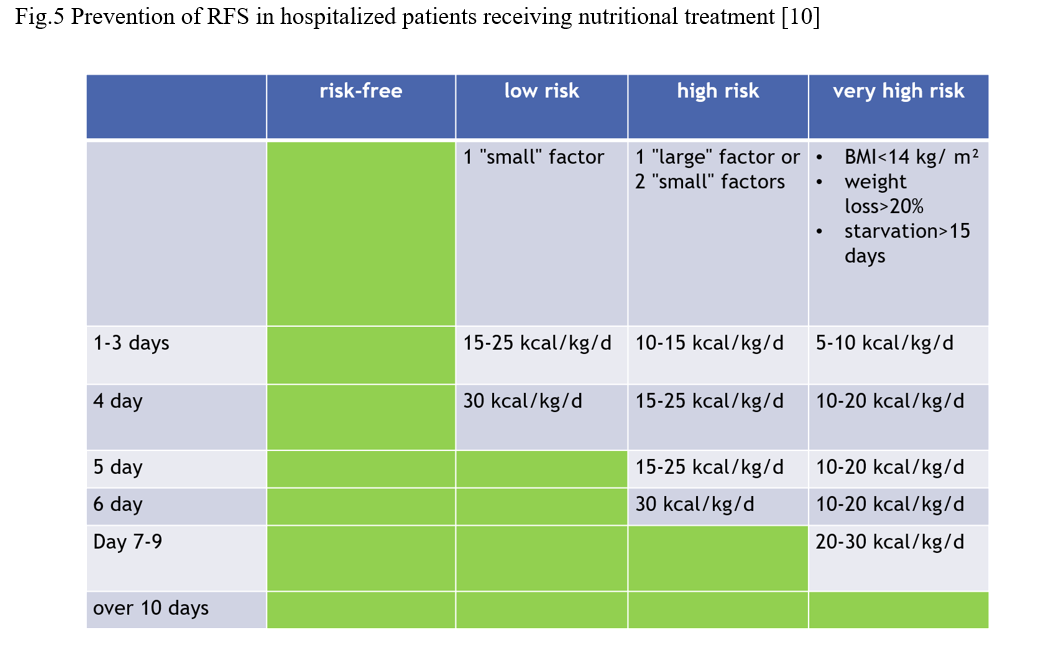
Figure 3
