Thyroid Adenocarcinoma with Papillary Growth
Thyroid Adenocarcinoma with Papillary Growth
Sai Sravya Sahini *
*Correspondence to: Sai Sravya Sahini, School of Health Sciences, The University of Georgia, Kostava St. 77a, 0171 Tbilisi, Georgia.
Copyright.
© 2024 Sai Sravya Sahini. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 13 September 2024
Published: 01 October 2024
Abstract
Papillary adenocarcinoma of the thymus is a very rare tumor worldwide, representing less than 1% of thymic primary carcinomas. Papillary morphology occurs more frequently in other types of carcinomas such as thyroid and lung cancer, so the diagnosis is challenging and the prognosis varies according to the histological lineage. There are few cases reported in the literature, and in Peru, no evidence has been found so far.
We present the case of a 58-year-old male with an intrathoracic tumor who, after being evaluated by the pulmonology and thoracic surgery department for 11 years due to chest pain and mild dyspnea, was diagnosed with a thymic carcinoma of the papillary adenocarcinoma type of very aggressive presentation. His diagnosis was confirmed by imaging studies and immunohistochemistry, and currently, he continues to receive treatment.
Keywords: Thymic carcinoma; papillary adenocarcinoma; mediastinal mass; thymus.
Thyroid Adenocarcinoma with Papillary Growth
Introduction
Primary thymic carcinomas are rare tumors that comprise approximately 0.06% of thymic neoplasms (1). They are classified into squamous cell carcinoma (90%), basaloid carcinoma (5%), mucoepidermoid carcinoma (2%), clear cell carcinoma (3%), adenocarcinoma (< 1%), among others (2). Papillary adenocarcinoma of the thymus is a very rare neoplasm, with few cases described worldwide. It was first described in 1988 and introduced in the World Health Organization (WHO) classification in 1999 (3). Its etiology occurs more frequently in other types of carcinomas such as thyroid and lung carcinomas, and it can subsequently metastastize to the thymus or mediastinum (4).
Papillary adenocarcinomas of the thymus often originate from tyoe A or AB thymomas, which are tumnors composed of solid oval-shaped epithelial cells, differentiated by the presence (AB) or absence (A) of immature T cells (TdT+) (5, 6). Patients may be asymptomatic or present symptoms related to the tumor lesion (chest pain, cough or dyspnea), or they are symptomatic. Imaging findings often show regional involvement and distant metastases are rare (7). Histopathologically, it consists of tubulopapillary structures lined with cuboidal to polygonal cells, atypically surrounding the oval nucleus; small nucleoli and eosinophilic or clear cytoplasm (5).
Immunophenotypic studies show that papillary adenocarcinoma of the thymus is positive for epithelial markers such as LeuM1 and BerEP4 and may be focally positive for CD5 and CEA. In contrast, it is negative for thyroid, lung and mesothelial organ-specific markers. The Ki67-proliferation index of tumor cells is less than 10% (3, 5). One of its differencial diagnoses is metastatic adenocarcinoma, so CD5 and CD117 positivity has been used to confirm thymic origin (8, 10, 11).
Because of the diagnosis of thymic carcinoma is challenging and even more so that of this papillary adenocarcinoma type variety, we report the case of a 58-years-old patient with mediastinal mass and metastastic disease, whose anatomopahological study resulted in papillary adenocarcinoma of the thymus.
Case Report
Male patient, 58 years old, native and from Cerro de Pasco. He had oncological family history of mother who died of tonsil cancer. He refers a time of illness of 11 years with a diagnosis of pulmonary cyst, of approximately 50 mm and, with surgical possibility; however, the patient refused surgery and only continued in controls. Such lesion was growing over the years, causing mild chest pain and, in recent months, mild dyspnea and weight loss, so he was referred in April 2016 to the Medical Oncology Service, bringing imaging tests that evidenced a mediastinal widening and a mediastinal mass (Figure 1A y 1B).
In May 2016, the patient was hospitalized in the Medical Oncology Service. He presented stable vital functions and underwent laboratory and imaging evaluations. He underwent a multislice spiral tomography of brain, thorax, abdomen and pelvis, evidencing a heterogeneous mass of 150mm x 100mm x 151mm in anterior mediastinum, infiltrating pleura and adjacent cortical bone, associated with nodes in internal mammary chain of 10mm, mediastinal adenopathies of 12mm, right pleural effusion and lytic lesions in cranial calotte, D10 – D12 and L3 – L5 (Figure 2). Also, a trucut biopsy of the mediastinal mass gave an anatomopathological diagnosis as papillary adenocarcinoma of thymus, and immunohistochemical tests were positive for Ki-67 (15%), CD5, CD117, and negative for p53, CK20, TTF-1 (Figure 3A).
For such reason, the patient received neoadjuvant chemotherapy with carboplatin 700 mg/paclitaxel 346 mg every 21 days for 6 cycles (Mayo 2016 – October 2016). In November, at the end of his sixth cycle of chemotherapy, the patient was reevaluated by a control tomography, evidencing an anterior and heterogeneous mediastinal mass, measuring 150mm x 110mm x145mm (approximately 1 cm larger than previous imaging), which did not infiltrate adjacent structures, associated with mediastinal adenopathies of 18mm, new lung nodules of 7 – 8mm, a right paravertebral formation of 23mm x 22mm, and a lytic lesion in D8, so it was classified as disease progression.
In December, after a Multidisciplinary Meeting of the Thorax Comitee, it was decided to perform a tumor cytoreduction, which revealed a large, highly vascularized thymic tumor adherent to the heart and large vessels. The anatomopathological result was papillary adenocarcinoma of thymus with positive immunohistochemistry for Ki-67 (10%) and pancytokeratin, and negative for p53, TTF-1. CD45, CK7 and CK20 (Figure 3B). During surgery, the patient presented hemodynamic instability and respiratory failure, so partial surgery was performed and he was transferred to Critical Care Unit, presenting favorable evolution. The patient was reevaluated after surgery (February 2017) by control tomographic scan (Figure 4) and evidencied disease progression, an anterior mediastinal mass measuring 140mm x 130mm x 115mm, associated to adjacent lymph node conglomerates, mild pleural effusion, retroperitoneal lymph node conglomerates, and lytic lesions in the cranial calotte, costal arches and D8.
It was decided to administer second-line chemotherapy treatment with cisplatin 115 mg/docetaxel 115 mg every 21 days for 6 cycles (February 2017 to June 2017). During the last cycle, the patient presented hip pain, so he was reevaluated by a tomographic scan, which showed stable disease for the mediastinal mass, but showed lytic lesions at the iliac bone and D8 and L3, which infiltrated the medullary canal, so the patient was treated with the administration of radiotherapy to lumbar spine with 50.4 Gy in 15 sessions. Finally, the patient continued in outpatient controls, with stable disease until now.
Figure 1: (A) PA chest X-ray (04/24/16) with widening of the mediastinum ocuppied by a radiopaque image. (B) Thorax tomographic scan (04/25/16) with mass-like imaging dependent on the mediastinum, no evidence of adequate cleveage plane.
Figure 2. Tomography at diagnosis of the disease (05/30/16). There is evidence of (A) lytic image in cranial calotte, (B) anterior mdediastinal mass of 150mm x 100mm x 151mm infiltrating adjacent structures, and (C) lytic lesions in costals arches y vertebrals bodies.
Figure 3. Anatomopathological findings. In the upper row, we observed the immunochemistry panel of the trucut biopsy of the tumor mass. From left & right (A) H&E staining, 400X objective; (B) TTF-1 (-); (C) CK7 (-); (D) CD117 (+); and (E) CD5 (+). In the lower row, we observed the immunohistochemistry panel of the surgical specimen. From left to right: (F) H&E staining, 400X objective; (G) TTF-1 (-); (H) CK7 (-); (I) CD45 (-); y (J) PANCK (+)
Figure 4. Postoperatory tomography scan (02/28/17). There is evidence of (A) new lytic lesion in cranial calotte, (B) anterior mediastinal mass of 140mm x130mm x 115mm that infiltrate adjacent structures and associated with lymph node conglomerates and right mild pleural effusion, and (C) lytic lesions in costals arches and vertebrals bodies.
Discussión
We present the clinicopathological features of a rare case of papillary adenocarcinoma of the thymus in a 58-years-old mal patient, whose age is within the ranges described in another studies. Initially diagnosed as a pulmonary cyst for 11 years, he was referred to our Service due to the presence of a mediastinal mass both in radiological and tomographical studies, after which he was staged by a tomographic scan, showing an anterior mediastinal mass of 15cm in diameter, with bone and lung metastases, he was treated with neoadjuvant chemotherapy for 6 cycles and then, was underwent to a tumor cytoreduction, which was only partial due to an adherence to the heart and large vessels. Given evidence of disease progression, he continued receiving adjuvant chemotherapy for 6 cycles, and given signs of spinal cord compression, after the last cycle, he received radiotherapy for 15 sessions, and is currently in controls. In 1998, Matsuno et al. (12) reported for the first time, four cases of papillary adenocarcinoma of the thymus in patients of average age (56 – 70 years), three cases were associated with spindle cell thymoma, and two of the three cases invaded the lung. On immunohistochemical studies, the tumor cells were positive for CEA, Leu M1, Ber EP4 and CD5, and negative for thyroglobulin and pulmonary surfactant apoprotein; the histological similarities and intimate association with spindle cell thymoma indicate that papillary thymic carcinoma may arise from tubulopapillary formations, sometimes seen in the primary tumor. When comparing the results of this report with ours, we observed that in our case there was no association with thymoma and immunohistochemistry ruled out any possibility of originating from another organ, with some limitations due to the lack of some markers; however, although our case showed a low proliferation index, it presented an aggressive behavior with multiple metastases, which is the reason of the importance to report this case.
Ten cases have been reported worldwide of papillary adenocarcinoma of the thymus, with variable clinical and pathological features (4). In general, the reported cases showed local recurrences and poor response to chemotherapy, however, with good survival, being the longest at 5 years, as reported by Matsuno et al. (12). Two cases associated with thymic cyst were reported (13, 15), of which the evolution is unknown in the case described by Yoshino et al. (15); in the case described by Zaitlin et al. (13), it is known that the patient died 26 months after diagnosis, presenting lung and bone dissemination. In our case, there was a disease time of 11 years with diagnosis of lung cyst, it could be possible that it was a mediastinal cyst (possibly of thymic origin) and that it developed later into a papillary adenocarcinoma of the thymus, which presented a progressive evolution. In the case described by Choi et al. (14), the patient had a papillary adenocarcinoma with many psammoma bodies and high nuclear grade, with immunohistochemical sitains similar to our case. In addition, Ibuki et al. (9) reported a case of papillary thymic adenocarcinoma with aggressive behavior, presenting infiltration to the sternum, for which he underwent to wide resection, without subsequent adjuvant treatment, presenting good survival. Our case, similarly to the previous one, presents stable disease. At present, the patient continues in controls.
In conclusion, we present a case of papillary adenocarcinoma of the thymus, featured by a low proliferation rate, but with aggressive behavior. The poor response to systemic therapy is not yet understood; however, the patient has good survival.
Table 1: Cases of papillary thymic adenocarcinoma reported in the literature
|
Author, Year |
Sex, Age |
Macroscopy |
Residual thymus |
Associated thymoma |
CD5 Reactivity |
Treatment |
Follow-up |
|
Matsuno et al, 1998 |
M, 70 |
Cyst, 8 x 5 x 3 cm |
Unknown |
Yes |
Focal |
Surgery; Radiotherapy,50 Gy; radiotherapy (first recurrence) |
Recurrence in lung hilum at 8 months, pleural issemination at 12 months |
|
Matsuno et al, 1998 |
F, 69 |
Mass, 5 x 3 x 1 cm |
Yes |
Yes |
No |
Wide surgical resection, including thymus, lung upper lobe and pleura |
Unknown |
|
Matsuno et al, 1998 |
F, 61 |
Mass, 10 x 8 x 5 cm |
No |
Yes |
Focal |
Surgery |
Died 7 months after, another causes |
|
Matsuno et al, 1998 |
M, 56 |
Mass, 3.5 x 3 x 1 cm |
Yes |
No |
No |
Neoadjuvant chemotherapy, carboplatin + VP-16; surgery; radiotherapy, 50.4 Gy |
Non-response to CT; recurrence-free at 5 years. |
|
Choi et al, 2003 |
M, 39 |
Mass, 4.5 x 4 x 2 cm |
No |
No |
Strong |
Neoadjuvant chemotherapy; wide resection; radiotherapy; surgery; CT y RT (recurrence) |
Non-response to CT; recurrence at 24 months; alive with active disease at 3 months |
|
Zaitlin et al, 2003 |
F, 51 |
Cyst, 12 x 9 x 8 cm |
Yes |
No |
No |
Partial surgical resection; CT; RT |
Local recurrence at 14 months; lung and vertebral issemination at 19 months; died at 26 months |
|
Yoshino et al, 2005 |
F, 29 |
Cyst, 5.5 x 5 x 4 cm |
Yes |
Yes |
No |
Surgery |
Unknown |
|
Furtado et al, 2010 |
M, 44 |
Mass, 3.5 x 3.5 x 3 cm |
Yes |
No |
No |
Surgery: tumoral resection; radiotherapy; chemotherapy |
Subcutaneous recurrence at 18 months; alive at 24 months with active disease |
|
Morikawa et al, 2010 |
F, 68 |
Cyst, 4 cm diameter |
No |
Yes |
No |
Surgery (VATS); chemotherapy |
Alive, non-recurrence at 15 months |
|
Ibuki et al, 2014 |
M, 64 |
Mass, 7.5 x 5.5 x 7.2 cm |
No |
No |
No |
Surgery: In bloc resection of the tumor, upper half of sternum, terminal ends of clavicles; wedge resection |
Alive, disease-free at 36 months |
References
1. Marchevsky AM, McKenna RJ, Jr., Gupta R. Thymic epithelial neoplasms: a review of current concepts using an evidence-based pathology approach. Hematol Oncol Clin North Am. 2008; 22: 543.
2. WHO. WHO Classification of Tumours of the Thymus. 3th Edition. International Agency for Research on Cancer, Lyon, 2000.
3. Matsuno Y, Rosai J, Shimosato Y. Papillary adenocarcinoma. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours Pathology & Genetics Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004: 183.
4. Furtado A, Nogueira R, Ferreria D, et al. Papillary Adenocarcinoma of the Thymus: Case Report and Review of the Literature. International Journal of Surgical Pathology 2010; 18 (6): 530-533.
5. OMS/IARC. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th Edition. International Agency for Research on Cancer, Lyon, 2015.
6. Marx A, Chan J, Coindre JM, Detterbeck F et al. The 2015 World Health Organization Classification of Tumours of the Thymus. J Thorac Oncol 2015; 10: 1383 – 1395.
7. Maghbool M, Ramzi M, Nagel I, et al. Primary adenocarcinoma of the thymus: an immunohistochemical and molecular study with review of the literature. BMC Clinical Pathology 2013, 13: 17.
8. Hosaka Y, Tsuchida M, Umezu H, Eimoto T, Hashimoto T et al. Primary thymic adenocarcinoma coexisting with type AB thymoma: a rare case with Kong-term survival. General Thoracic Cardiovascular Surgery 2010; 58: 488-491, discussion 491-482.
9. Ibuki et al. Primary papillary carcinoma of the thymus with invasión into subcutaneous tissue through the sternum. Journal of Cardiothoracic Surgery 2014; 9: 77.
10. Weissferdt A, Moran CA. Thymic carcinoma part 1: a clinicopathologic and immunohistochemical study of 65 cases. American Journal of Clinical Pathology 2012; 138: 103-114.
11. Weissferdt A, Moran CA. Thimic carcinoma part 2: a clinicopathologic correlation of 33 cases with a proposed staging system. American Journal of Clinical Pathology 2012; 138: 115.121.
12. Matsuno Y, et al. Papillary carcinoma of the thymus: report of four cases of a new mycroscopic type of thymic carcinoma. Am J Surg Pathol 1998; 22(7): 873 – 80.
13. Zaitlin N, et al. Papillary Adenocarcinoma in a Thymic Cyst: A Pitfall of a Thoracoscopic Excision. Ann Thorac Surg 2003; 76: 1279 -81.
14. Choi WW, et al. Adenocarcinoma of the thymus: report of two cases, including a previously undescribed mucinous subtype. Am J Surg Pathol 2003; 27 (1): 124 – 130.
15. Yoshino M, et al. Pappillary Carcinoma of the Thymus Gland. Ann Torac Surg 2005; 80: 741 – 742.
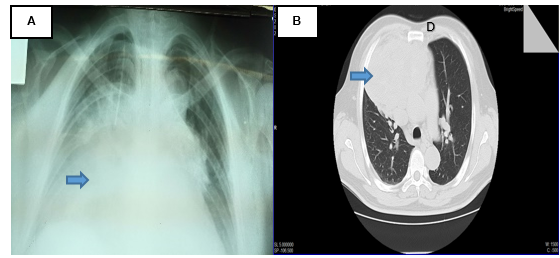
Figure 1
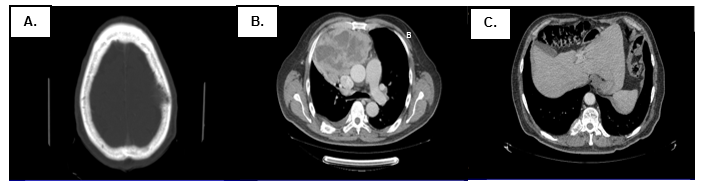
Figure 2
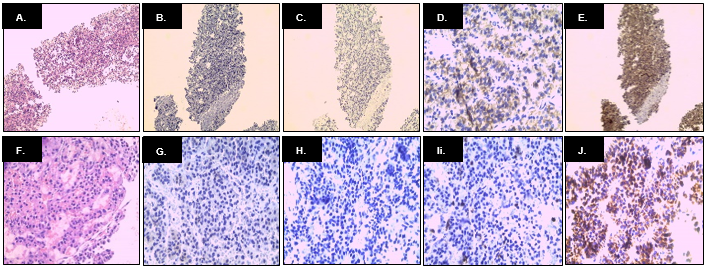
Figure 3
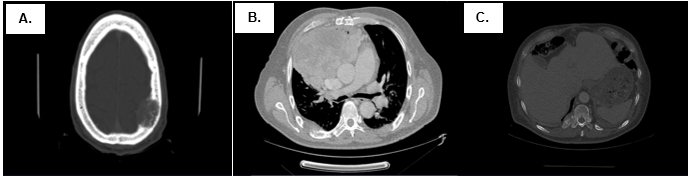
Figure 4
