Recent Developments in RSV Prophylaxis
Recent Developments in RSV Prophylaxis
Dr. Olga Tzetzi, MD, PhD *
*Correspondence to: Dr. Olga Tzetzi, MD, PhD, General paediatrician, private practice, Thessaloniki, Greece. President of the Association of private practice paediatricians in Northern Greece.
Copyright
© 2024: Dr. Olga Tzetzi. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 22 February 2024
Published: 01 October 2024
Recent Developments in RSV Prophylaxis
Introduction
RSV is a highly transmissible seasonal virus which can be responsible, in some cases, for unpredictable and potentially severe outcomes in infants. RSV is a frequent cause of respiratory disease, which can include bronchiolitis and/or pneumonia, in infants and is a leading cause of hospitalizations, most of which are in otherwise healthy infants born at term. By the age of two years, almost all children have been infected, but natural infection does not provide long – lasting immunity. Reinfection is common but usually affects only the upper respiratory tract. Children below five years of age (particularly infants below six months), and adults over 65 years of age are the most affected by RSV-associated severe disease.
Figure 1
RSV causes about 58.000 to 80.000 hospitalizations and 100 to 300 deaths per year in children under 5 years, according to data from CDC. Up to 3% of children in their first year of life are hospitalized due to RSV infection each year in the US. In the EU, an average of 245.244 yearly hospital admissions with a respiratory infection per year were associated with RSV in children under the age of 5. Historically, a preventive option for the broad infant population has not been available, but newer strategies and approaches could potentially change the RSV disease prevention landscape and help towards the implementation of national RSV prevention programs.
On the 17th of July 2023 the U.S. Food and Drug Administration approved Beyfortus (nirsevimab-alip) for the prevention of Respiratory Syncytial Virus (RSV) lower respiratory tract disease in neonates and infants born during or entering their first RSV season, and in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
Beyfortus is a monoclonal antibody with activity against RSV. One dose of Beyfortus, administered as a single intramuscular injection prior to or during RSV season, may provide protection during the RSV season.
The safety and efficacy of Beyfortus were supported by three clinical trials (Trials 03, 04 and 05). The key measure of efficacy was the incidence of medically attended RSV lower respiratory tract infection (MA RSV LRTI), evaluated during the 150 days after Beyfortus administration. MA RSV LRTI included all health care provider visits (physician office, urgent care, emergency room visits and hospitalization) for lower respiratory tract disease with worsening clinical severity and a positive RSV test. Trials 03 and 04 were randomized, double-blind, placebo-controlled, multicenter clinical trials.
Trial 03 included 1,453 preterm infants (born at greater than or equal to 29 weeks of gestational age up to less than 35 weeks of gestation) who were born during or entering their first RSV season. Of the 1,453 preterm infants in the trial, 969 received a single dose of Beyfortus and 484 received placebo. Among infants who were treated with Beyfortus, 25 (2.6%) experienced MA RSV LRTI compared with 46 (9.5%) infants who received placebo. Beyfortus reduced the risk of MA RSV LRTI by approximately 70% relative to placebo.
For Trial 04, the primary analysis group within the trial included 1,490 term and late preterm infants (born at greater than or equal to 35 weeks in gestational age), 994 of whom received a single dose of Beyfortus and 496 of whom received placebo. Among infants who were treated with Beyfortus, 12 (1.2%) experienced MA RSV LRTI compared with 25 (5.0%) infants who received placebo. Beyfortus reduced the risk of MA RSV LRTI by approximately 75% relative to placebo.
Trial 05, a randomized, double-blind, active palivizumab -controlled, multicenter trial, supported the use of Beyfortus in children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season. The trial enrolled 925 preterm infants and infants with chronic lung disease of prematurity or congenital heart disease. The safety and pharmacokinetic data from Trial 05 provided evidence for the use of Beyfortus to prevent MA RSV LRTI in this population.
Figure 2
On the 15th of August 2023, American Academy of paediatrics recommended all infants under 8 months receive nirsevimab to protect them from RSV, while also provided guidance for continued use of palivizumab in the 2023 -24 season. Nirsevimab is recommended for all infants under 8 months born during or entering their first RSV season. It also should be given to children 8 months through 19 months who are at increased risk of severe RSV disease and entering their second RSV season. This high-risk group includes children with chronic lung disease of prematurity who require medical support during the six months before the start of the second RSV season, children who are severe immunocompromised, children with cystic fibrosis who have manifestations of severe lung disease or weight-for-length below the 10th centile.
Typical RSV season is October through March. Infants born shortly before or during the RSV season should receive nirsevimab in their first week of life either in the hospital or an outpatient setting. Newborns with a prolonged hospital stay should get it shortly before or after discharge. For other infants and eligible toddlers, nirsevimab should be administered shortly before the start of the RSV season. Age- eligible infants and toddlers who did not receive a dose at the start of the season can receive a dose at any time during the season. Only eligible high-risk children should get a dose in both their first and second seasons, even if they are younger than 8 months entering their second season. However, a healthy infant born at the end of the season who did not receive nirsevimab and is less than 8 months entering their second RSV season may receive nirsevimab. However, nirsevimab may not be available to administer this season. In these cases, eligible high – risk infants and children in their first or second year of life should receive the monoclonal antibody palivizumab, which includes a series of monthly doses. Children who receive fewer than five doses of palivizumab in the 2023 -24 season can receive one dose of nirsevimab, but then should not receive any additional doses of palivizumab. Any children who receive nirsevimab should not receive palivizumab later that season. High-risk children who received palivizumab in their first RSV season should receive nirsevimab in their second season, if it is available and they remain eligible. If it is unavailable, they should receive palivizumab.
Figure 3
On the 21st of July, EMA has recommended granting a marketing authorisation in the European Union (EU) for Abrysvo, the first RSV vaccine indicated for passive immunization of infants from birth through 6 months of age following administration of the vaccine to the mother during pregnancy. This vaccine is also indicated for active immunization of adults aged 60 years and older, given the fact that RSV infection can also be serious for that age, as it can cause acute respiratory infection, influenza-like illness or community-acquired pneumonia. Abrysvo is a bivalent vaccine composed of two recombinant RSV fusion surface glycoproteins selected to optimise protection against RSV A and B strains. These proteins are essential for RSV to infect the body and are also the main targets of the antibodies generated to fight the infection.
The opinion by EMA’s human medicines committee is based on data from two randomised, placebo-controlled, pivotal studies.
In one study, 3,695 individuals at 24-36 weeks pregnant were administered Abrysvo while 3,697 received a placebo (dummy injection). The assessment showed that the vaccine was effective in reducing both severe medically attended lower respiratory tract illness and medically attended lower respiratory tract illness occurring within 180 days after birth.
In the other study, 18,488 adults aged 60 years and older were administered the vaccine, while 18,479 received a placebo. The results of the study demonstrated efficacy for Abrysvo in the reduction of RSV-associated lower respiratory tract illness with 2 (or more) symptoms and with 3 (or more) symptoms.
The most common side effects reported in individuals between 24 and 36 weeks pregnant were vaccination site pain, headache and muscle pain. In individuals 60 years of age and older the most frequently reported side effect was vaccination site pain.
The opinion adopted by the CHMP is an intermediary step on Abrysvo’s path to patient access. The opinion will now be sent to the European Commission for the adoption of a decision on an EU-wide marketing authorisation. Once a marketing authorisation has been granted, decisions about price and reimbursement will take place at the level of each Member State, taking into account the potential role/use of this medicine in the context of the national health system of that country.
On May 2023, FDA approved the world’s first RSV vaccine for adults 60 years and older, Arexvy. According to the U.S. Centers for Disease Control and Prevention, each year in the U.S., RSV leads to approximately 60,000-120,000 hospitalizations and 6,000-10,000 deaths among adults 65 years of age and older. Hospitalizations for adults in the EU, Norway and the United Kingdom are on average 158.000 per year.
The safety and effectiveness of Arexvy is based on the FDA’s analysis of data from an ongoing, randomized, placebo-controlled clinical study conducted in the U.S. and internationally in individuals 60 years of age and older. The main clinical study of Arexvy was designed to assess the safety and effectiveness of a single dose administered to individuals 60 years of age and older. Participants will remain in the study through three RSV seasons to assess the duration of effectiveness and the safety and effectiveness of repeat vaccination. Data for a single dose of Arexvy from the first RSV season of the study were available for the FDA’s analysis.
In this study, approximately 12,500 participants have received Arexvy and 12,500 participants have received a placebo. Among the participants who have received Arexvy and the participants who have received a placebo, the vaccine significantly reduced the risk of developing RSV-associated LRTD by 82.6% and reduced the risk of developing severe RSV-associated LRTD by 94.1%.
Among a subset of these clinical trial participants, the most commonly reported side effects by individuals who received Arexvy were injection site pain, fatigue, muscle pain, headache and joint stiffness/pain. Among all clinical trial participants, atrial fibrillation within 30 days of vaccination was reported in 10 participants who received Arexvy and 4 participants who received placebo.
In two other studies, approximately 2,500 participants 60 years of age and older received Arexvy. In one of these studies, in which some participants received Arexvy concomitantly with an FDA-approved influenza vaccine, two participants developed acute disseminated encephalomyelitis (ADEM), a rare type of inflammation that affects the brain and spinal cord, seven and 22 days, respectively, after receiving Arexvy and the influenza vaccine. One of the participants who developed ADEM died. In the other study, one participant developed Guillain-Barré syndrome (a rare disorder in which the body’s immune system damages nerve cells, causing muscle weakness and sometimes paralysis) nine days after receiving Arexvy.
In conclusion, the anonymous vote by agency advisors in favour of approving the above medicines for infants and older people is a giant forward in the fight of RSV and a testament to the relentless hardwork of researchers and clinicians worldwide.
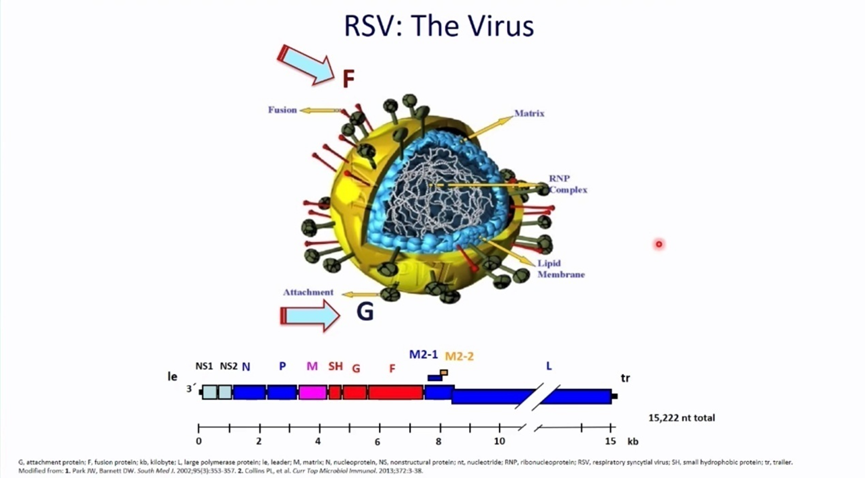
Figure 1
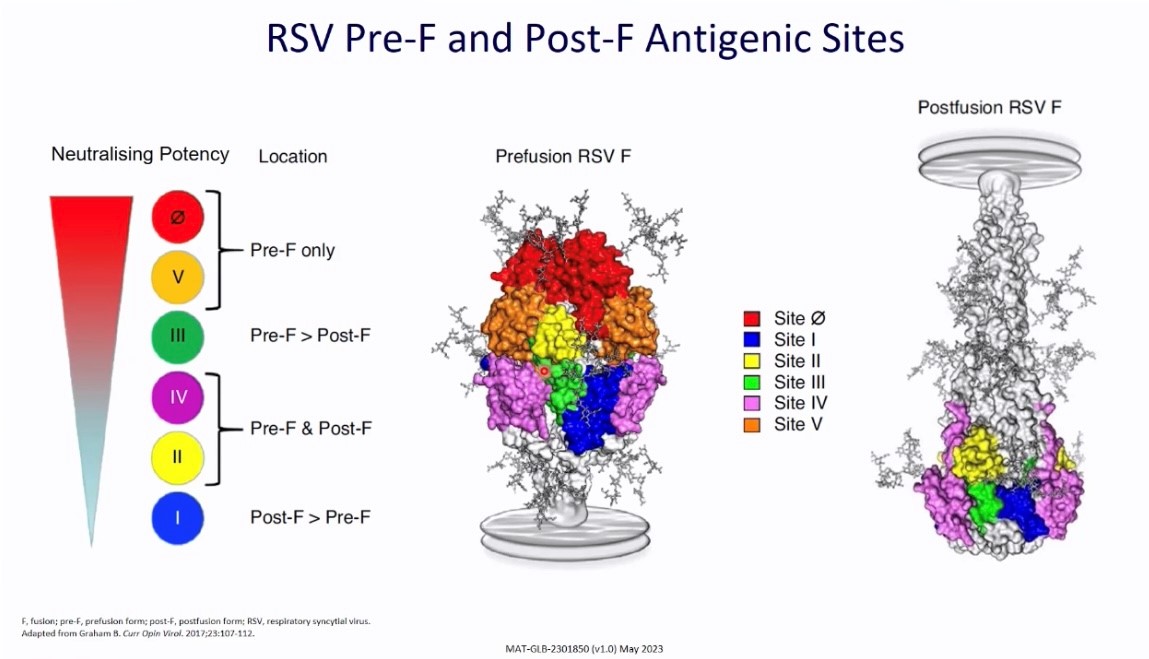
Figure 2

Figure 3
