Management of Bioanalytical Samples in BA/BE Studies: Challenges Faced.
Management of Bioanalytical Samples in BA/BE Studies: Challenges Faced.
Mr. Chandra Shekar Reddy Ambati*1; Dr. Kaushal Kapadia2
1. Texila American University
2 Clinical research professional.
*Correspondence to: Mr. Chandra Shekar Reddy Ambati, Texila Americal University.
Copyright © 2024: Mr. Chandra Shekar Reddy Ambati. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 16 Sept 2024
Published: 01 Oct 2024
DOI: https://doi.org/10.5281/zenodo.13892743
Abstract
The design, performance and evaluation of bioavailability and bioequivalence have evolved over the last few years substantially. The bioavailability (BA) and bioequivalence (BE) study findings are crucial to know the safety and tolerability of generics against corresponding innovator drugs; to ensure they are comparable and safe for consumption by human subjects. The BA/BE study process is streamlined by applicable regulatory guidelines or requirements worldwide. In this regard a gold standard has already been established by global agencies like USFDA, EMA, CDSCO etc., who have produced extensive and detailed guidance documents adhering to GCP guidelines.
This study aims to examine the practical on-the-field issues and challenges in processing the bioanalytical samples collected in BA/BE studies. The issues or drawbacks in the existing practices and their impact on the quality and consistency of pharmacokinetic data shall be explored in this study. Following that, we tried to understand the perspective and opinions to make improvements in the process, from the experts in the field of BA/BE studies who are currently working in the industry and who have faced and overcame the bottlenecks. While sponsors are required to submit the BA/BE data before any further clinical research and/or drug development process, it is crucial to understand the importance of the accuracy of these data. The reason being, that those values are the basis to assess the rate and extent of absorption of a particular active pharmaceutical ingredient in a drug, be it a NCE or generic one.
In this study, special attention has been attributed to instrumentation adopted during BA/BE studies. Calibration of the instrument is crucial to maintain accuracy and precision throughout the analysis, while ionization efficiency affects the sensitivity and detection limits of the method. The importance of accurate mass measurements is emphasized, as it impacts the identification and quantification of analytes.
In conclusion, the quality of BA/BE data that will be used in clinical research is influenced by multiple factors, including sample preparation, chromatographic separation, instrumental considerations, data acquisition parameters, and data processing. A comprehensive understanding of the impacting factors is essential for optimizing the safe and appropriate conduct of BA/BE study, ensuring robust and reliable results in clinical research applications.
Management of Bioanalytical Samples in BA/BE Studies: Challenges Faced.
Introduction
Recent estimates by the World Health Organization (WHO) state that 95% of the global human population is living with one or more health conditions. An estimated 421 million hospitalizations occur annually worldwide, with approximately 42.7 million of them leading to adverse events. This current situation of public health requires accelerated development of the medical sector, which involves adopting novel treatment regimens and more effective drugs. More research is focused on discovering and developing drugs aimed to treat the million patients living with medical conditions.
Inadequate pharmaceuticals are a worldwide issue that impact both developed and developing nations. The proliferation of fake medications is a concern for governments and health authorities since it puts patients at risk and provides funding for illegal activity. It is widely acknowledged that subpar and fake medications pose a global issue that impacts both developed and developing nations. The most difficult realization is that counterfeit medications have frequently resulted in major side effects, even fatalities, for users. These are grave repercussions that heighten the urgency of the war on fake medications.
Over the last century many undesired cases have emerged involving fake and substandard drugs. In Nigeria, fake vaccines were blamed for 2,500 deaths in 1995. 89 deaths in Haiti and 30 deaths in India between 1995 and 1998 were attributed to the use of paracetamol preparations containing diethylene glycol. Thirty people in Cambodia lost their lives in 1999 as a result of taking a fake antimalarial drug made with sulphadoxine-pyrimethamine (World Health Organization, 2006). Due to the high cost of real drugs and lax government oversight, developing nations are an obvious target for counterfeiters, but developed nations may also be on the radar. In 2006, anti-flu drugs worth approximately £500,000 were seized by UK authorities (Mukhopadhyay, 2007). The Food and Drug Administration (FDA) declared in 2012 that a falsified anti-cancer medication had been discovered in the United States. There have been multiple reports of fake medicines being in circulation globally.
The United States’ Food and Drug Administration (FDA) has defined counterfeit medication as “contaminated or contain the wrong or no active ingredient. They could have the right active ingredient but at the wrong dose”. Counterfeit medications don't meet the innovator's standard preparation requirements for quality, safety, and efficacy or have the appropriate amount of active ingredients (European Medicine Agency, 2012; Food and Drug Administration, 2012b). Drugs that are subpar or counterfeit may find their way onto the market. This can happen to both generic and name-brand medications. A drug is considered substandard if it contains more or less of the active ingredient than what is required in the formulation (Johnston and Holt, 2014).
The Food and Drug Administration (2003a) states that bioequivalence is considered to be present when the ratio of the target pharmacokinetic parameters, area under the curve (AUC) to maximum concentration (Cmax), falls within the range of 0.8-1.25 (80-125%) with 90% confidence intervals (CI). Similarly should be the time to maximum plasma concentration (tmax). Drug development relies heavily on bioequivalence studies to create novel drug formulations and their generic equivalents. In the event that manufacturing changes occur after approval, these studies become even more crucial (European Medicines Agency, 2010). Guidelines have been established in numerous countries for the approval of generic medications. In addition to other pharmaceutical information, generic manufacturers are required to submit proof of bioequivalence tests, but not data from clinical trials or preclinical tests, which are drawn-out and costly processes (European Medicines Agency, 2008). There may be some slight variations between brand-name and generic medications during bioequivalence testing. For instance, a generic medication may differ from the brand-named version in terms of size, shape, or color (Kesselheim et al., 2008).
Methodology
challenges and perspectives on factors affecting the quality of bioanalytical samples in BA-BE research conducted. The Questionnaires was developed with the help of available literature and by understanding the issues and bottlenecks in handling samples collected for bioanalytical testing necessary in BA/BE studies.
This study was conducted in a qualitative exploratory model in order to understand, inform, and explore the possible issues faced in practical scenario of BA/BE sample collection. Without abundant existence of supporting past research literature, a fairly closed-ended approach in asking questions was adopted for the challenges section, while the opinions and perspectives in improvement strategies were gathered through an open-ended approach. The chosen research design was aligned with the previously stated research The research project was conducted in a survey-based model where we gathered information on
and the reliability and accuracy of the data. A validated questionnaire was shared with clinical research
professionals experienced in bioavailability-bioequivalence studies, with whom the survey was objectives, allowing for in-depth exploration, interpretation, and analysis of the subjective aspects and contextual influences related to the factors affecting the quality of BA/BE data and understanding the impact of sample-handling process on those.
Results
bioanalytical samples in BA/BE studies. In response, 79.95% of the participants responded in negative, while only 20.05% stated that they had indeed faced challenges. When those who had faced these challenges were asked, if documentation for study subject, sample collection or processing were missing at some point, the responses were almost equally distributed with 49.41% saying ‘yes’ and 50.58% saying ‘no’
Figure 1
Next the participants were asked if they had ever shipped samples for BA/BE analysis to another facility. To this, majority of the participants (88.92%) stated ‘yes’, while only 11.08% said ‘no’. Those who said ‘yes’ were then asked the question, ‘were the Documentation for study sample shipping Times for each samples missing or inconsistent’. To which, all of them said ‘no’, meaning that no documentation was When the participants were asked, if they had faced challenges in the management and processing of missing
Figure 2
The participants were then required to respond to the following question asking if they had faced challenges in primary processing of samples and getting it ready for analysis as per the regulatory requirements for BA/BE. 71.23% of the participants (302 of 424) responded negatively and 28.77% of the participants said ‘yes’ to the question.
Figure 3
Next the participants were asked if they had faced challenges in selecting active components for BA/BE studies. In response to this, 95.28% stated that they have not faced any such challenges, while 4.72% stated that they indeed have faced challenges
Figure 4
Discussions
As evident from the age and years of experience of participants involving BA/BE studies, it can be seen that care was taken to include people who had substantial experience on the matter and were credible sources of information. The average work experience of the participants was of 6 years while the average age of the participants was 37, despite the oldest participant being of 52 years of age. This lowering of average age can occur from the choice of medium for conducting the survey. As the questionnaire was circulated through an online platform, it might have faced limited circulation, availability of internet may appear as an issue in engaging personnel involved in BA/BE studies.
Another drawback that appeared was the dominance of participants from one particular geographical area. As can be seen from the result, most of the participants stated that their research was being conducted under the regulatory guidance of the CDSCO, which holds jurisdiction over Indian institutions. This does affect in harmonization of data across different guiding agencies. However, apart from CDSCO, there were participants from other organizations like the US-FDA and EMEA. It should be noted that in general, establishing a local market can be helpful in addressing the issue of geographical diversity. Local production can assist the organization in reducing the length of their supply chain, avoiding currency fluctuations, and comprehending the demands and requirements of the particular market. Additionally, it can assist in addressing the nation's pressing demands.
Variations in regulations exist among these different guidelines. In case of India, the study's test product needs to be an accurate representation of the one that will be sold. Unless there is an exception, the test product should typically come from a batch of at least 100,000 units or 1/10 of the production scale. The ability of the product and procedure to be produced on an industrial scale should be highly assured by the batches that are produced. A single dosage, non-replicate cross-over design investigation is required. Depending on how the composition of the various test product strengths relates to each other, it might be adequate to establish BA/BE at only one strength—typically the highest—if many strengths are applied for (Wadakkan & Rajagopal, 2019). It is advised that BE research using immediate release and modified release dosage forms use single dose, non-replicate cross-over designs. For fed BE studies where the test and reference formulations are compared after a test meal, the United States Food and Drug Administration (USFDA) typically recommends a single-dose, two-period, two-treatment, two-sequence cross research design (Shaik et al., 2011). When comparing two formulations, the European Medicines Agency (EMA) advises using a randomized, two-period, two-sequence single dose crossover design. However it should be
noted that in response to the present questionnaire majority of the participants stated that the guideline/regulations followed in their region do designate best practices for BA/BE studies separately. Neither any separate best practices are designated for processing and storage of study samples in these guidelines, according to most of the participants. This appears to be a key aspect which must be adapted in future updates of the aforementioned guidelines.
While conducting such BA/BE studies, one cannot simply overlook the host of challenges involved. The majority of bioanalytical laboratories now employ LC/MS/MS as their main analytical technique when conducting analyses for human bioavailability (BA)/bioequivalence (BE) investigations because of its better sensitivity and selectivity. Several Food and Drug Administration (FDA) guidance documents, the Compliance Program Guidance Manual, Good Laboratory Practice (GLP) rules, and other publications have published the compliance requirements for creating, validating, and employing LC/MS/MS (James et al., 2004, Shah et al., 2000). Owing to this and other guidelines, it became apparent in the present study that most of the participants had not faced any challenges in the management and processing of bioanalytical samples in BA/BE studies. However, those who did face such issues, were equally divisive in their responses regarding the documentation involved in cases where sample collection and/or processing at certain points were missing or inconsistent. This can rise from lack of experience in facing such rare occurrences. There seemed to be ample experience involved among participants regarding overseas transport of bioanalytical samples, as most of the participants stated that they have indeed handled such transports. Those who had experience in such transfer were also unanimous in stating that they have not encountered any situation where documentation had gone missing or was not available.
Majority of the participants in the present study were of the opinion that their country’s regulatory authority does not consider different storage recommendations for different dosage forms before evaluation of systemic exposure profile of a test drug product compared to that of a reference drug product (BE study). A thorough review of literature on the matter also failed to reveal any such clear recommendation regarding storage of samples (Kaushal et al. 2016, Nagadurga 2019). Therefore inclusion of such specifications is very much warranted in future versions of international guidelines. Availability of additional test methods other than pharmacokinetic methods was also investigated, from which it became clear that in regard to BA/BE studies the pharmacokinetic approach was the most favored approach among all those who were involved. For more than 20 years, the pharmaceutical industry and national regulatory bodies have embraced the idea of BA/BE, which is used for both new and generic drugs. This has led to the availability of thousands of affordable, high-quality generic medications anywhere in the world. However, evaluating BA/BE is not an easy task, and a lot of research has been done recently to provide fresh, more efficient methods for evaluating BE. Despite the advancement of the pharmacokinetic approach regarding BA/BE studies, other methods should not be overlooked, as these methods can be quite useful in cross-verification.
Conclusion
For more than 20 years, national regulatory agencies and the pharmaceutical industry around the world have embraced the BE concept. As a result, the pharmaceutical industry has produced and sold hundreds of generic medications following regulatory approval. Many developments in the last few years have led to the development of different methods for evaluating BE through research that would guarantee high-quality, interchangeable, and reasonably priced medications. Still, there is much work to be done.
In the present research work, important insight was generated regarding the personnel who are directly involved in BA/BE studies. It was noted that, there is a strong and urgent requirement of including best practice guidelines involving sample collection and processing prior to adopting bioanalytical approaches. The importance of generating study-specific training for the staff and development of more sensitive and selective analytical methods is warranted. This serves as the completion of the primary objective of the thesis, which was to understand the management and challenges faced in processing of bioanalytical samples of BA and BE studies in clinical research.
References
1. Ahmed, T. and Rohatagi, S., 2012. Application of LC-MS in supporting PK/PD studies during drug discovery and development. Pharm. Res, 5, pp.2514-2526.
2.Andersson, T., Hassall, E., Lundborg, P., Shepherd, R., Radke, M., Marcon, M., Dalväg, A., Martin, S., Behrens, R., Koletzko, S. and Becker, M., 2000. Pharmacokinetics of orally administered omeprazole in children. Official journal of the American College of Gastroenterology| ACG, 95(11), pp.3101-3106.
3.Blackstone, E.A., Fuhr Jr, J.P. and Pociask, S., 2014. The health and economic effects of counterfeit drugs. American health & drug benefits, 7(4), p.216.
Mr. Chandra Shekar Reddy Ambati, (2024). Management of Bioanalytical Samples in BA/BE Studies:
Challenges Faced. MAR Pathology and Clinical Research, 02(03).
Mr. Chandra Shekar Reddy Ambati, MAR Pathology and Clinical Research (2024) 2:3 Page 11 of 12
4.Boehm, G., Yao, L., Han, L. and Zheng, Q., 2013. Development of the generic drug industry in the US after the Hatch-Waxman Act of 1984. Acta Pharmaceutica Sinica B, 3(5), pp.297-311.
5.Covey, T., 1996. Liquid chromatography/mass spectrometry for the analysis of protein digests. Protein and peptide analysis by mass spectrometry, pp.83-99.
6.Devanshu, S., Rahul, M., Annu, G., Kishan, S. and Anroop, N., 2010. Quantitative bioanalysis by LC-MS/MS: a review. Journal of pharmaceutical and biomedical sciences, 7(7).
7.Green, J.M., 1996. Peer reviewed: a practical guide to analytical method validation. Analytical chemistry, 68(9), pp.305A-309A.
8.James, C.A., Breda, M. and Frigerio, E., 2004. Bioanalytical method validation: a risk-based approach?. Journal of pharmaceutical and biomedical analysis, 35(4), pp.887-893.
9.Johnston, A. and Holt, D.W., 2014. Substandard drugs: a potential crisis for public health. British journal of clinical pharmacology, 78(2), pp.218-243.
10.Kaushal, N., Singh, S.K., Gulati, M., Vaidya, Y. and Kaushik, M., 2016. Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: impact on generic drug substitution. Journal of Applied Pharmaceutical Science, 6(4), pp.206-222.
11.Kelesidis, T. and Falagas, M.E., 2015. Substandard/counterfeit antimicrobial drugs. Clinical microbiology reviews, 28(2), pp.443-464.
12.Liang, B.A., Mackey, T.K. and Lovett, K.M., 2013. Illegal “no prescription” internet access to narrow therapeutic index drugs. Clinical therapeutics, 35(5), pp.694-700.
13.Midha, K.K. and McKay, G., 2009. Bioequivalence; its history, practice, and future. The AAPS journal, 11, pp.664-670.
14.Shaik M, Thirunagari BL, Sathe A, 2011. The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies- An overview. Comparative Effectiveness Research 1: 1-25.
15.Shrank, W.H., Cox, E.R., Fischer, M.A., Mehta, J. and Choudhry, N.K., 2009. Patients’ perceptions of generic medications. Health affairs, 28(2), pp.546-556.
Mr. Chandra Shekar Reddy Ambati, (2024). Management of Bioanalytical Samples in BA/BE Studies:
Challenges Faced. MAR Pathology and Clinical Research, 02(03).
Mr. Chandra Shekar Reddy Ambati, MAR Pathology and Clinical Research (2024) 2:3 Page 12 of 12
16.Snyder, A.P., 1996. Electrospray: a popular ionization technique for mass spectrometry.
17.Thomson, B.A., 1998. Atmospheric pressure ionization and liquid chromatography/mass spectrometry—together at last. Journal of the American Society for Mass Spectrometry, 9(3), pp.187-193.
18.Ulmer, C.Z., Koelmel, J.P., Jones, C.M., Garrett, T.J., Aristizabal?Henao, J.J., Vesper, H.W. and Bowden, J.A., 2021. A review of efforts to improve lipid stability during sample preparation and standardization efforts to ensure accuracy in the reporting of lipid measurements. Lipids, 56(1), pp.3-16.
19.Wadakkan, J.W. and Rajagopal, S.S., 2019. An overview on bioequivalence regulatory requirements of orally administered pharmaceutical products: The US-FDA guidelines and gulf cooperation council guidelines. Journal of Pharmacy Research, 13(1), p.53.
20.Yadav, P., 2015. Health product supply chains in developing countries: diagnosis of the root causes of underperformance and an agenda for reform. Health systems & reform, 1(2), pp.142-154.
Medtronic
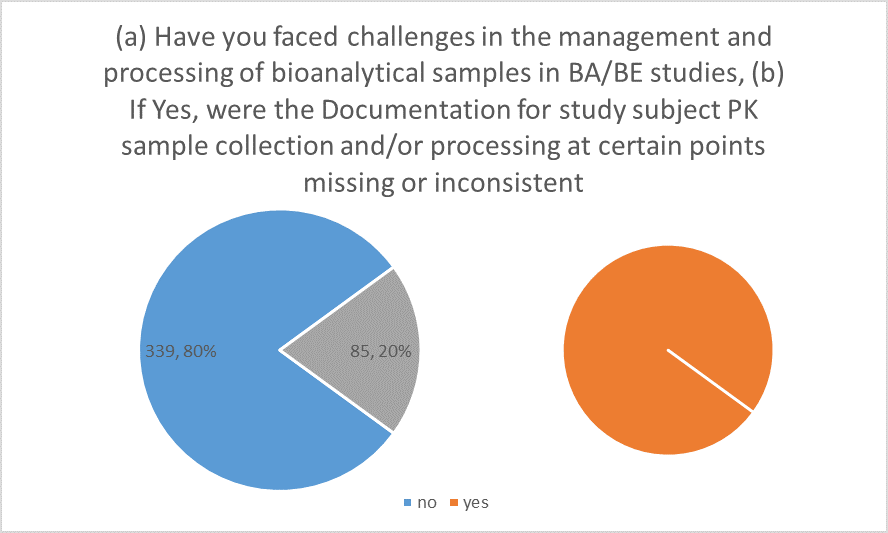
Figure 1
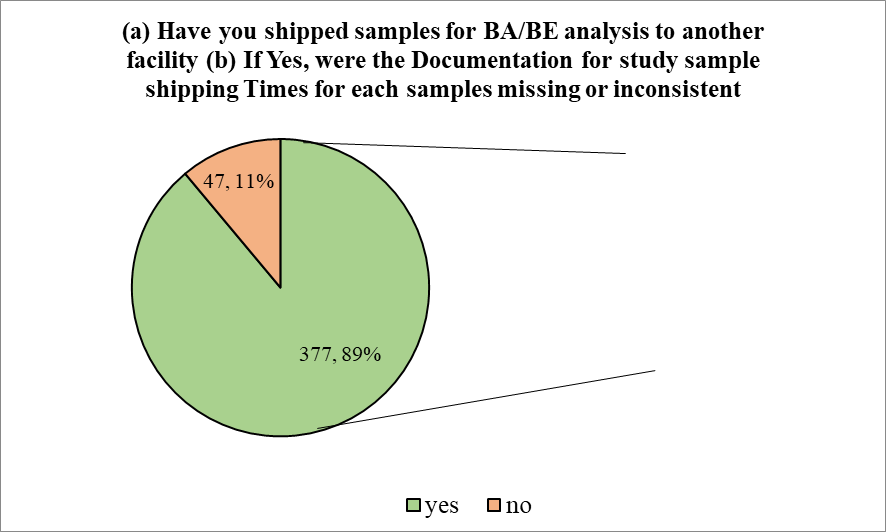
Figure 2
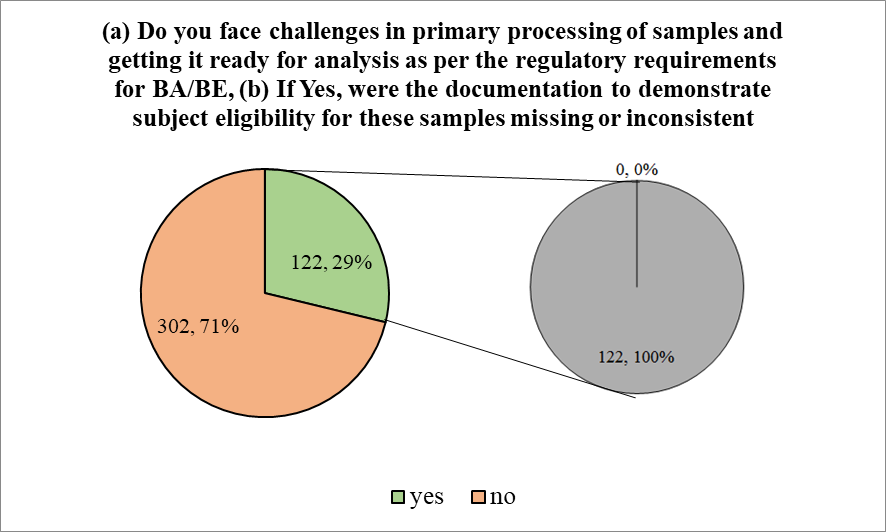
Figure 3
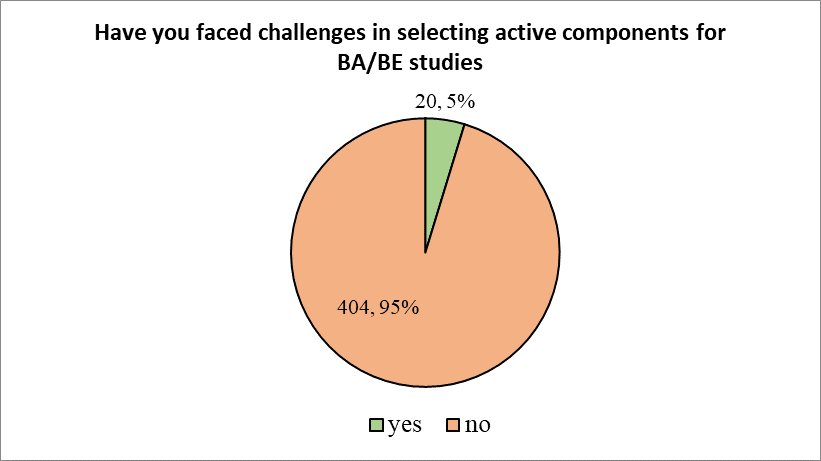
Figure 4
