Quality Management of Bioanalytical Samples in BA/BE Studies
Quality Management of Bioanalytical Samples in BA/BE Studies
Mr. Chandra Shekar Reddy Ambati*1; Dr. Kaushal Kapadia2
1 Texila Americal University.
2 Clinical research professional.
*Correspondence to: Mr. Chandra Shekar Reddy Ambati, Texila Americal University.
Copyright
© 2024: Mr. Chandra Shekar Reddy Ambati. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 16 Sept 2024
Published: 01 Oct 2024
Abstract
The design, performance and evaluation of bioavailability and bioequivalence have evolved over the last few years substantially. The bioavailability (BA) and bioequivalence (BE) study findings are crucial to know the safety and tolerability of generics against corresponding innovator drugs; to ensure they are comparable and safe for consumption by human subjects. The BA/BE study process is streamlined by applicable regulatory guidelines or requirements worldwide. In this regard a gold standard has already been established by global agencies like USFDA, EMA, CDSCO etc., who have produced extensive and detailed guidance documents adhering to GCP guidelines.
This study aims to examine the practical on-the-field issues and challenges in processing the bioanalytical samples collected in BA/BE studies. The issues or drawbacks in the existing practices and their impact on the quality and consistency of pharmacokinetic data shall be explored in this study. Following that, we will try to understand the perspective and opinions to make improvements in the process, from the experts in the field of BA/BE studies who are currently working in the industry and who have faced and overcame the bottlenecks. While sponsors are required to submit the BA/BE data before any further clinical research and/or drug development process, it is crucial to understand the importance of the accuracy of these data. The reason being, that those values are the basis to assess the rate and extent of absorption of a particular active pharmaceutical ingredient in a drug, be it a NCE or generic one.
In this study, special attention has been attributed to instrumentation adopted during BA/BE studies. Calibration of the instrument is crucial to maintain accuracy and precision throughout the analysis, while ionization efficiency affects the sensitivity and detection limits of the method. The importance of accurate mass measurements is emphasized, as it impacts the identification and quantification of analytes.
In conclusion, the quality of BA/BE data that will be used in clinical research is influenced by multiple factors, including sample preparation, chromatographic separation, instrumental considerations, data acquisition parameters, and data processing. A comprehensive understanding of the impacting factors is essential for optimizing the safe and appropriate conduct of BA/BE study, ensuring robust and reliable results in clinical research applications.
Quality Management of Bioanalytical Samples in BA/BE Studies
Introduction
In 2011, a news report highlighted the issue of counterfeit medicines in Yemen, with millions of patients falling victims to expired smuggled, forged and substandard medications. A different TV report examined fake medications in West Africa. China, India, and Pakistan produced counterfeit medications, which were then shipped to developing nations. Patients suffered blindness and died as a result of this. The rise in counterfeit and inferior medications can be attributed to inadequate government oversight and unsuitable storage environments. Following such reports WHO had advised increased supervision of the medications, further verification and checks with drug manufacturers should be made before administration (World Health Organization, 2015).
There are multiple routes by which a drug can be administered to a patient. Among these the most efficient and the quickest route is through intravenous injection. However, this can cause some discomfort to the patient. In case of such instances, the most preferred route for drug delivery by both patients and healthcare professionals is the oral route. Drugs administered through this route provides the most convenience to patients experiencing chronic conditions. During the procedure of discovering novel drugs, it is a basic requirement for compounds under scrutiny to be effective in adequately achieve systemic exposure after oral intake. This is usually measured by plasma or blood concentrations with respect to time. In case of an oral compound, the most important property is to deliver the active substance to its target site in an amount sufficient to elicit the desired pharmacologic response. This is of particular importance as even though a compound exhibits strong effects on the pharmacologic target in vitro, its beneficial pharmacologic effects may not materialize if it is poorly absorbed and results in low plasma concentrations after an oral dose. It is extremely important in case of the administered drug. Achieving adequate systemic absorption is crucial in attaining the intended pharmacologic effect, but there are also significant clinical and financial justifications for optimizing oral administration. Along with the research endeavor and the great amount of effort behind the multiple phases of clinical trials it is of paramount importance that the integrity of the samples involved is not compromised in any way. Therefore the challenges arising in processing of bio analytical samples involved in bioavailability and bioequivalence studies of drugs and their subsequent management is of particular importance to the entire research fraternity.
Bioavailability is defined as the rate and extent to which a particular drug substance reaches the systemic circulation. This is a very important factor in drug therapy, which can, in principle affect how a patient responds to a particular drug. Bioavailability of a drug can display variations based on a variety of reasons.
Such reasons may include patient related factors or dosage dependent factors. Patient related factors like age, underlying condition, genetic variation and gastrointestinal physiology are usually controllable. However, there are other factors which are partially controllable, such as the time of drug administration relative to meals referred to as stomach emptying time effect, the co-administration of other drugs which may affect absorption along with the compliance of the patient with the medication.
Bioequivalence between two formulations has been defined by multiple experts. Two pharmaceutical products are considered to be bioequivalent when their bioavailabilities, from the same molar dose, are so similar that they are unlikely to produce clinically relevant differences in therapeutic and/or adverse effects. Two products are considered to be therapeutically equivalent if they deliver the identical active moiety from the same molar dose and have similar efficacy and safety (Cartwright et al., 1991). The similarity in how brand-name and generic medications function has also been described as bioequivalence. In his definition of bioequivalence, Birkett stated that two pharmaceutical products are considered bioequivalent if they are pharmaceutically equivalent and if their bioavailabilities—the rate and extent of availability—after administration in the same molar dose are similar enough to be expected to have essentially the same effects in terms of both efficacy and safety (Birkett, 2003).
Methodology
The research project was conducted in a survey-based model where we would be gathering information on challenges and perspectives on factors affecting the quality of bioanalytical samples in BA-BE research and the reliability and accuracy of the data. A validated questionnaire will be shared with clinical research professionals experienced in bioavailability-bioequivalence studies, with whom the survey will be conducted. The Questionnaires was developed with the help of available literature and by understanding the issues and bottlenecks in handling samples collected for bioanalytical testing necessary in BA/BE studies.
This study was conducted in a qualitative exploratory model in order to understand, inform, and explore the possible issues faced in practical scenario of BA/BE sample collection. Without abundant existence of supporting past research literature, a fairly closed-ended approach in asking questions was adopted for the challenges section, while the opinions and perspectives in improvement strategies were gathered through an open-ended approach. The chosen research design was aligned with the previously stated research objectives, allowing for in-depth exploration, interpretation, and analysis of the subjective aspects and contextual influences related to the factors affecting the quality of BA/BE data and understanding the impact of sample-handling process on those.
Results
The participants were asked if they use pharmacokinetic approaches to assess equivalence of the absorption of a drug substance in a BE study, or they have other options involving (i) comparative bioavailability (bioequivalence) studies in biological fluid such as plasma, blood or urine, (ii) comparative pharmacodynamic studies in humans, (iii) comparative clinical trials, (iv) in-vitro dissolution tests. The participants were allowed to choose more than one option. 91.04% of the participants i.e. 386 of 424, stated that they always use pharmacokinetic approach, while 245 participants added that all types of test methods are utilized, as appropriate. 122 participants stated that they never use any in-vitro methods, while only 43 of the participants stated that such “Options” are only used for BE of imported drugs. It must be mentioned that, as participants were allowed to choose multiple options in response to this question, so there were participants who chose more than one option.
Figure 1
The next question looked into the technical aspect of conducting BA/BE studies. The participants were asked, ‘Do you think the data acquisition parameters have an impact on the quality and comprehensiveness of liquid chromatography-mass spectrometry (LC-MS) data in clinical research’. In response to which 50.47% of the participants stated ‘yes’, while 36.79% of participants chose ‘no’ as an answer. Only 12.74% of the participants stated that they don’t know.
Figure 2
The participants were then asked if they have faced any challenges related to extraction of sample analytes from the biological matrix. To which, majority of the participants i.e. 88.92% responded in negative. Only 11.08% of participants stated that they indeed have faced such challenges. Inquiring down the same line, the following question, ‘Have you encountered issues, inconsistency or discrepancy in the sample collection and processing forms regarding criteria for processing (centrifuge time, temperature & condition such as hemolysis, etc.) or storage (transfer time to freezer)?’ was asked to the participants. To which, a majority of 75.71% answered in positive, while the remaining 24.29% said that they have not come across any such issue.
Figure 3, 4
The participants were then required to answer the question, ‘Have you encountered any problems regarding the choice of stationary phase and mobile phase in chromatography’. In response, most of the participants (65.09%) stated that they did not know about it, while 28.77% of participants stated that they have not encountered any such problems. Only 26 out of 424 (6.13%) participants noted that they indeed have faced such scenarios.
Discussions
While conducting such BA/BE studies, one cannot simply overlook the host of challenges involved. The majority of bioanalytical laboratories now employ LC/MS/MS as their main analytical technique when conducting analyses for human bioavailability (BA)/bioequivalence (BE) investigations because of its better sensitivity and selectivity. Several Food and Drug Administration (FDA) guidance documents, the Compliance Program Guidance Manual, Good Laboratory Practice (GLP) rules, and other publications have published the compliance requirements for creating, validating, and employing LC/MS/MS (James et al., 2004, Shah et al., 2000). Owing to this and other guidelines, it became apparent in the present study that most of the participants had not faced any challenges in the management and processing of bioanalytical samples in BA/BE studies. However, those who did face such issues, were equally divisive in their responses regarding the documentation involved in cases where sample collection and/or processing at certain points were missing or inconsistent. This can rise from lack of experience in facing such rare
occurrences. There seemed to be ample experience involved among participants regarding overseas transport of bioanalytical samples, as most of the participants stated that they have indeed handled such transports. Those who had experience in such transfer were also unanimous in stating that they have not encountered any situation where documentation had gone missing or was not available.
Majority of the participants in the present study were of the opinion that their country’s regulatory authority does not consider different storage recommendations for different dosage forms before evaluation of systemic exposure profile of a test drug product compared to that of a reference drug product (BE study). A thorough review of literature on the matter also failed to reveal any such clear recommendation regarding storage of samples (Kaushal et al. 2016, Nagadurga 2019). Therefore inclusion of such specifications is very much warranted in future versions of international guidelines. Availability of additional test methods other than pharmacokinetic methods was also investigated, from which it became clear that in regard to BA/BE studies the pharmacokinetic approach was the most favored approach among all those who were involved. For more than 20 years, the pharmaceutical industry and national regulatory bodies have embraced the idea of BA/BE, which is used for both new and generic drugs. This has led to the availability of thousands of affordable, high-quality generic medications anywhere in the world. However, evaluating BA/BE is not an easy task, and a lot of research has been done recently to provide fresh, more efficient methods for evaluating BE. Despite the advancement of the pharmacokinetic approach regarding BA/BE studies, other methods should not be overlooked, as these methods can be quite useful in cross-verification.
While considering the pharmacokinetic approach the importance of LC/MS as a tool cannot be overlooked. The risk involved in developing new drugs is extremely great.
Countless lead compounds are produced and examined.
It's possible that only one lead compound out of every 100,000 is put through clinical testing. Out of ten lead candidates, only one is signed up and promoted. The fact that just one in three marketed medications recovers the R&D expenditure adds insult to injury to this high degree of failure. As a result, there is strong incentive to continuously assess drug development efforts and incorporate novel strategies that offer benefits for quicker development and more affordable research. This drive has caused the pharmaceutical sector to look for fresh chances including analysis and quick development. Recently, the mechanistic elements of ionization (Bruins, 1998) and the necessity to solve challenges that led to the development and use of LC/MS-based techniques (Snyder, 1996; Covey, 1996; Thomson, 1998) have been reviewed. A strong belief that the combination would be exceptional and offer significant advantages for analysis drove efforts to create and improve an interface for integrating a flowing liquid HPLC system into a high vacuum mass spectrometry environment (Arpino et al., 1981). A distinctive combination for pharmacological analysis was made possible by the vision and combined efforts of a broad group of trailblazing researchers.
In relation to the important role played by LC-MS and other pharmacokinetic approaches, the participants of the present survey were in majority regarding the seriousness of data acquisition parameters having an impact on the quality and comprehensiveness of liquid chromatography-mass spectrometry (LC-MS) data. As more than half of the participants were of the opinion that this is crucial, much care must be adopted during data acquisition. Coming down to sample handling issues at their end most stated that they have not encountered any challenges in extraction of sample analytes from biological matrices. However, majority of the participants stated that they have encountered issues, inconsistency or discrepancy in the sample collection and processing forms regarding criteria for processing (centrifuge time, temperature & condition such as hemolysis, etc.) or storage (transfer time to freezer). And more than half of the participants were found to be unaware of problems regarding the choice of stationary phase and mobile phase in chromatography. They were also found to be mostly unaware of difficulties in analyzing and processing the data obtained from your bioanalytical studies. It should be noted here that, the recommendations for bioanalytical methods that are utilized for the quantitative determination of pharmaceuticals and their metabolites in biological matrices such plasma, urine, and preclinical investigations are known as the Bioanalytical Method Validation (BMV) guidelines for industry. All of the steps required to show that a specific technique created and applied for the quantitative measurement of analytes in a given biological matrix is dependable and repeatable are included in the validation of bioanalytical methods (Green 1996). The process of determining if a bioanalytical method's performance characteristics satisfy the needs of the planned bioanalytical application is known as validation. The parameters for the validation of the bioanalytical method are used to express these performance characteristics. The primary validation factors for bioanalytical methods include recovery, stability, repeatability, sensitivity, and precision and accuracy. Nonetheless, a number of techniques, such as the freeze-thaw method, benchtop stability, long-term stability, and short-term temperature stability studies, can be used to assess the method's stability (Devanshu et al., 2010).
Conclusion
For more than 20 years, national regulatory agencies and the pharmaceutical industry around the world have embraced the BE concept. As a result, the pharmaceutical industry has produced and sold hundreds of generic medications following regulatory approval. Many developments in the last few years have led to the development of different methods for evaluating BE through research that would guarantee high-quality, interchangeable, and reasonably priced medications. Still, there is much work to be done.
In the present research work, important insight was generated regarding the personnel who are directly involved in BA/BE studies. It was noted that, there is a strong and urgent requirement of including best practice guidelines involving sample collection and processing prior to adopting bioanalytical approaches. The importance of generating study-specific training for the staff and development of more sensitive and selective analytical methods is warranted. This serves as the completion of the primary objective of the thesis, which was to understand the management and challenges faced in processing of bioanalytical samples of BA and BE studies in clinical research.
References
1.Arpino, P.J., Krien, P., Vajta, S. and Devant, G., 1981. Optimization of the instrumental parameters of a combined liquid chromatograph-mass spectrometer, coupled by an interface for direct liquid introduction: 2.II. Nebulization of Liquids by Diaphragms. Journal of Chromatography A, 203, pp.117-130.
3.Blackstone, E.A., Fuhr Jr, J.P. and Pociask, S., 2014. The health and economic effects of counterfeit drugs. American health & drug benefits, 7(4), p.216.
4.Boehm, G., Yao, L., Han, L. and Zheng, Q., 2013. Development of the generic drug industry in the US after the Hatch-Waxman Act of 1984. Acta Pharmaceutica Sinica B, 3(5), pp.297-311.
5.Bruins, A.P., 1998. Mechanistic aspects of electrospray ionization. Journal of Chromatography A, 794(1-2), pp.345-357.
6.Covey, T., 1996. Liquid chromatography/mass spectrometry for the analysis of protein digests. Protein and peptide analysis by mass spectrometry, pp.83-99.
7.Devanshu, S., Rahul, M., Annu, G., Kishan, S. and Anroop, N., 2010. Quantitative bioanalysis by LC-MS/MS: a review. Journal of pharmaceutical and biomedical sciences, 7(7).
8.Green, J.M., 1996. Peer reviewed: a practical guide to analytical method validation. Analytical chemistry, 68(9), pp.305A-309A.
9.Haas, J.S., Phillips, K.A., Gerstenberger, E.P. and Seger, A.C., 2005. Potential savings from substituting generic drugs for brand-name drugs: medical expenditure panel survey, 1997–2000. Annals of internal medicine, 142(11), pp.891-897.
10.James, C.A., Breda, M. and Frigerio, E., 2004. Bioanalytical method validation: a risk-based approach?. Journal of pharmaceutical and biomedical analysis, 35(4), pp.887-893.
11.Johnston, A. and Holt, D.W., 2014. Substandard drugs: a potential crisis for public health. British journal of clinical pharmacology, 78(2), pp.218-243.
12.Kaushal, N., Singh, S.K., Gulati, M., Vaidya, Y. and Kaushik, M., 2016. Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: impact on generic drug substitution. Journal of Applied Pharmaceutical Science, 6(4), pp.206-222.
13.Kaza, M., Kara?niewicz-?ada, M., Kosicka, K., Siemi?tkowska, A. and Rudzki, P.J., 2019. Bioanalytical method validation: new FDA guidance vs. EMA guideline. Better or worse?. Journal of pharmaceutical and biomedical analysis, 165, pp.381-385.
14.Kelesidis, T. and Falagas, M.E., 2015. Substandard/counterfeit antimicrobial drugs. Clinical microbiology reviews, 28(2), pp.443-464.
15.Liang, B.A., Mackey, T.K. and Lovett, K.M., 2013. Illegal “no prescription” internet access to narrow therapeutic index drugs. Clinical therapeutics, 35(5), pp.694-700.
16.Nagadurga, D.H., 2019. Bioavailability and bioequivalence studies. In Pharmaceutical Formulation Design-Recent Practices. London, UK: IntechOpen.
17.Shah, V.P., Midha, K.K., Findlay, J.W., Hill, H.M., Hulse, J.D., McGilveray, I.J., McKay, G., Miller, K.J., Patnaik, R.N., Powell, M.L. and Tonelli, A., 2000. Bioanalytical method validation—a revisit with a decade of progress. Pharmaceutical research, 17, pp.1551-1557.
18.Shaik M, Thirunagari BL, Sathe A, 2011. The basic regulatory considerations and prospects for conducting bioavailability/bioequivalence (BA/BE) studies- An overview. Comparative Effectiveness Research 1: 1-25.
19.Shrank, W.H., Cox, E.R., Fischer, M.A., Mehta, J. and Choudhry, N.K., 2009. Patients’ perceptions of generic medications. Health affairs, 28(2), pp.546-556.
20.Snyder, A.P., 1996. Electrospray: a popular ionization technique for mass spectrometry.
21.2018. Overview of the European Medicines Agency's Development of Product?Specific Bioequivalence 22.Guidelines. Clinical Pharmacology & Therapeutics, 104(3), pp.539-545.
23.Thomson, B.A., 1998. Atmospheric pressure ionization and liquid chromatography/mass spectrometry—together at last. Journal of the American Society for Mass Spectrometry, 9(3), pp.187-193.
24.Ulmer, C.Z., Koelmel, J.P., Jones, C.M., Garrett, T.J., Aristizabal?Henao, J.J., Vesper, H.W. and Bowden, J.A., 2021. A review of efforts to improve lipid stability during sample preparation and standardization efforts to ensure accuracy in the reporting of lipid measurements. Lipids, 56(1), pp.3-16.
25.Wadakkan, J.W. and Rajagopal, S.S., 2019. An overview on bioequivalence regulatory requirements of orally administered pharmaceutical products: The US-FDA guidelines and gulf cooperation council guidelines. Journal of Pharmacy Research, 13(1), p.53.
26.White, C.M., 2021. Counterfeit drugs: a major issue for vulnerable citizens throughout the world and in the United States. Journal of the American Pharmacists Association, 61(1), pp.e93-e98.
27. Yadav, P., 2015. Health product supply chains in developing countries: diagnosis of the root causes of underperformance and an agenda for reform. Health systems & reform, 1(2), pp.142-154.
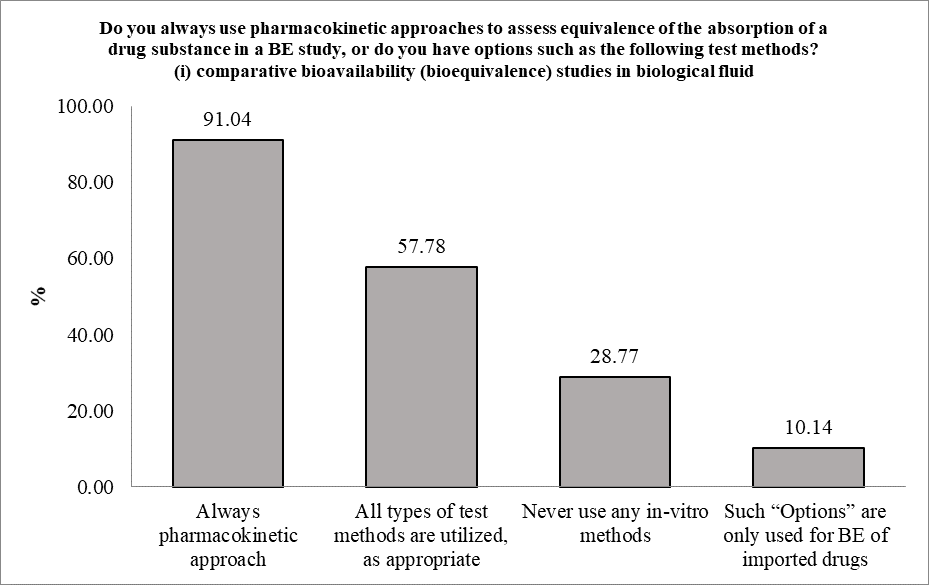
Figure 1
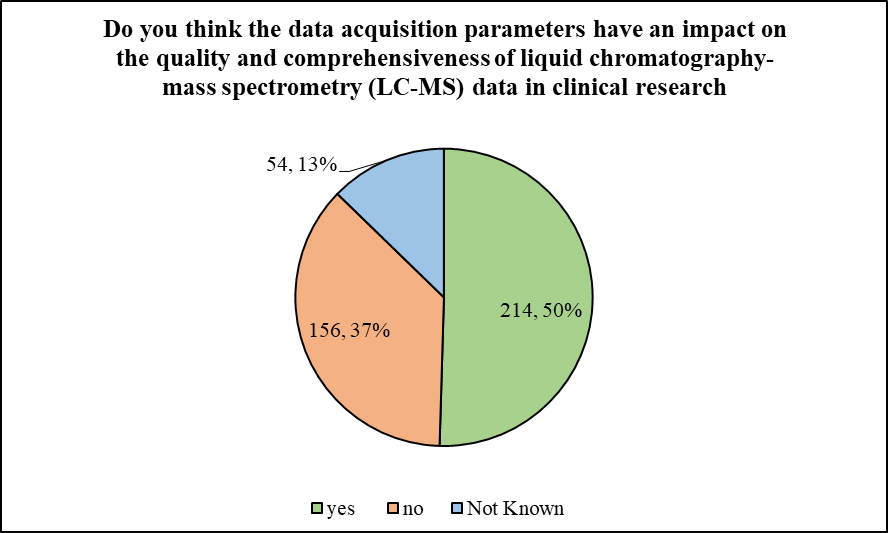
Figure 2
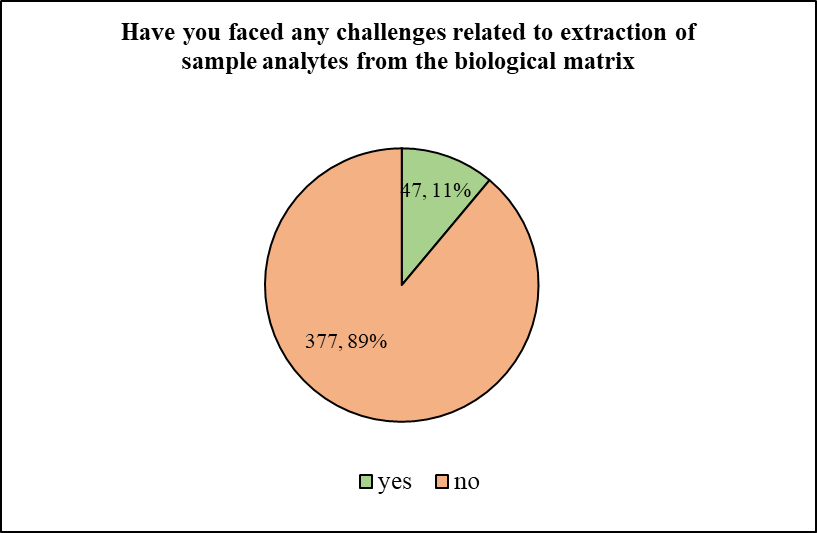
Figure 3
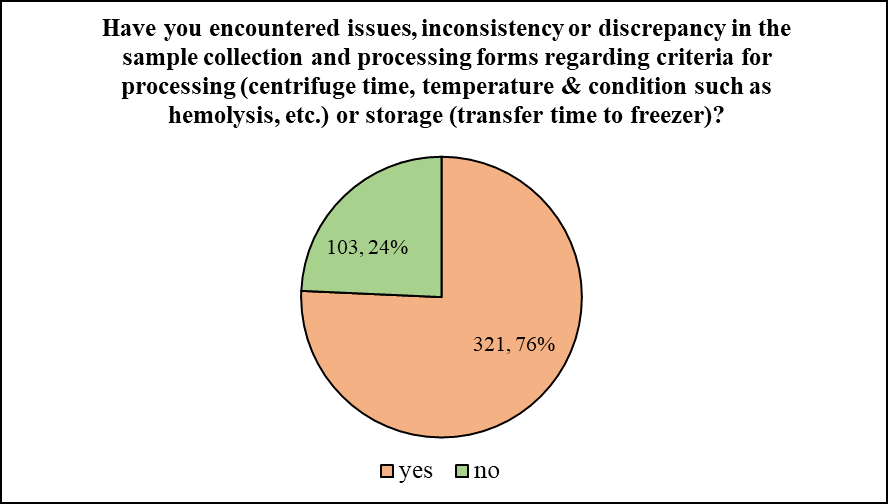
Figure 4
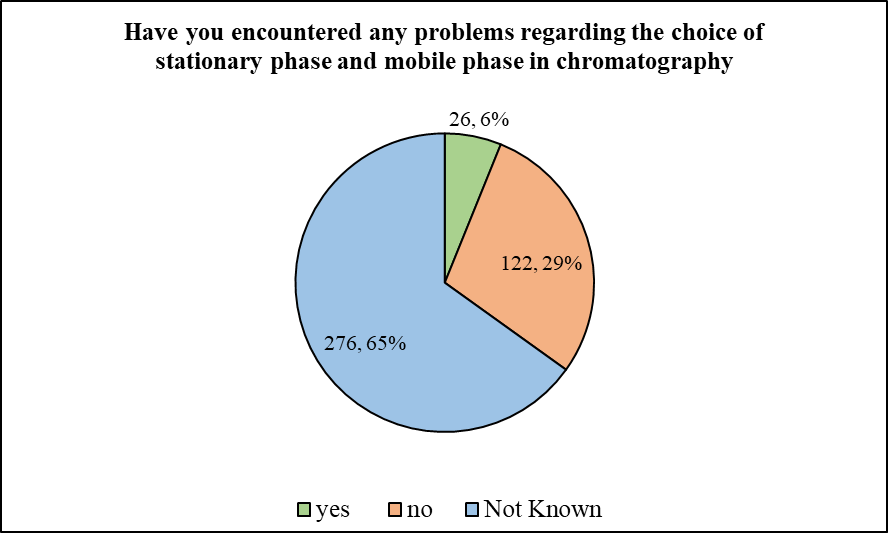
Figure 5
