Transient Thrombocytopenia and Bilateral Immature Cataract in a Newborn with Down’s Syndrome
Transient Thrombocytopenia and Bilateral Immature Cataract in a Newborn with Down’s Syndrome
Mohammad Shahbaz Alam 1*, Devendrasing Jadhav2, Sofia Abdul Razak Fakih3, Suvas Chand4
1. Mohammad Shahbaz Alam, Department of Neonatology, Zulekha Hospital, Dubai, United Arab Emirates.
2. Devendrasing Jadhav, Department of Neonatology, Zulekha Hospital, Dubai, United Arab Emirates.
3. Sofia Abdul Razak Fakih, Department of Neonatology, Medeor Hospital, Dubai, United Arab Emirates.
4. Suvas Chand, Department of Neonatology, Zulekha Hospital, Dubai, United Arab Emirates.
*Correspondence to: Mohammad Shahbaz Alam, Department of Neonatology, Zulekha Hospital, Dubai, United Arab Emirates.
Devendrasing Jadhav, Department of Neonatology, Zulekha Hospital, Dubai, United Arab Emirates.
Copyright
© 2024: Mohammad Shahbaz Alam, Devendrasing Jadhav . This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 Oct 2024
Published: 20 Oct 2024
Abstract
Down’s syndrome (DS) is the most common viable form of trisomy. Among the various haematological abnormalities observed in neonates with Down’s syndrome, thrombocytopenia is a fairly common presentation in the first few weeks of life. Numerous ocular manifestations have also been discovered in such neonates including strabismus, nasolacrimal duct obstruction, keratoconus, eyelid abnormalities, optic nerve abnormalities, retinal abnormalities, and cataract. This case report describes a rare combination of transient thrombocytopenia and bilateral congenital cataract in a male neonate with DS. This particular combination of clinical findings is one of the first to be reported in any case of DS, to the best of our knowledge.
Transient Thrombocytopenia and Bilateral Immature Cataract in a Newborn with Down’s Syndrome
Introduction
Down’s syndrome (DS) is the most common viable form of trisomy (Trisomy 21). It has an incidence of 1 in 700-800 live births, with the occurrence of thrombocytopenia being as high as the general population.1 Various haematological abnormalities including neutrophilia, thrombocytopenia and polycythaemia have been discovered among the newborns with Down’s syndrome with the incidence of 80%, 66% and 34% respectively.2,3,4 Out of 66% of newborns with thrombocytopenia, 6% of them have platelet counts <50,000/cubic millilitre, in their first week of life.5 Thrombocytopenia in non-DS neonates has been extensively researched and various underlying contributory mechanisms causing this condition have been documented but the exact mechanism in DS neonates is still unknown. However suboptimal response to thrombopoietin and chronic fetal hypoxia leading to intrauterine growth restriction are postulated to be two major causes of thrombocytopenia in such neonates.6
Facial dysmorphism and hypotonia are the most striking features associated with Down’s syndrome, however numerous ophthalmologic manifestations have also found to be present including strabismus, nasolacrimal duct obstruction, keratoconus, eyelid abnormalities, cataract, optic nerve abnormalities and retinal abnormalities. 7,8,9,10 Our case also described an interesting finding of bilateral immature cataract in association with Down’s syndrome. Although the presence of cataract is not very uncommon, Rahi and Dezateux et al have reported a cohort of patients with bilateral congenital or infantile cataract in DS neonates and suggested higher incidence of this phenomenon in Down’s patients as compare to general paediatric population.11
We report a newborn with Down’s syndrome with transient thrombocytopenia and bilateral immature cataract. To the best of our knowledge, this is the first case reported globally manifesting both these rare findings in a single female neonate with DS. This emphasizes the need of detailed clinical examination including red reflex assessment and a complete hemogram even if no bleeding manifestation is present.
Case Presentation
A term girl neonate was born vaginally to nonconsanguineous Asian parents with maternal age being 41 years and paternal age being 39 years. They had 2 previous healthy children, admitted to Neonatal Intensive Care Unit (NICU) after birth in view of cyanosis, tachypnoea and not meeting target saturation at room air, which required some respiratory support and discharged in 48 hours. Mother was booked to a private hospital at 6 weeks of gestation. There was no history of chronic illness, fever, or rash in the first and second trimesters. She was offered for Non-invasive prenatal testing (NIPT) and Double marker testing, but parents refused for the same. However, nuchal translucency done at around 14 weeks was 2.8 mm. Anomaly scan and Group B Streptococci screening were normal, but mother developed fever three weeks before delivery for which she was given antibiotics and she recovered well with the treatment.
Anthropometric examination of the baby was suggestive of birth weight of 3860 grams, length of 50 cm and head circumference of 35 cm (all within 10-90th centiles). Head to toe examination revealed bilateral corneal opacities and facial appearance suggestive of Down’s phenotype including, round face, low set ears, depressed nasal bridge, simian crease on both palms, saddle crease, saddle gap on both feet, generalized hypotonia and a small skin tag over the lower neck in the midline. This tag was later confirmed with ultrasound as dermal in origin without any underlying thyroid connection.
Vital signs displayed normal heart rate and rhythm. Central and peripheral pulses were well felt. However, the newborn appeared cyanotic with respiratory rate of 60-70 breaths per minute. Preductal saturation was noted in the range of 70-80% at room air. He was managed with a flow of 3 litres/ minute and fractional oxygen of 30% to achieve a target saturation of 95% using heated humidified high flow nasal cannula. On examination of chest, there was equal bilateral air entry on auscultation, no retractions, and no adventitious sound. Abdominal examination revealed no organomegaly. Examination of cardiovascular system was essentially normal with normally heard S1 S2 with no murmur. Echocardiography performed in view of cyanosis and desaturations exhibited large patent ductus arteriosus (PDA) of 6mm with bidirectional shunt while a later follow up Echocardiography done after a week showed closing PDA with reduction in size to 2 mm. Baby required low flow oxygen support till first week of life with a few failures of oxygen free trial. Initial X-ray was suggestive of mild pulmonary plethora attributed to PDA. Feeds were started on day 2 and escalated to day appropriate feed volume by day 4 of life but it took 3 more days to achieve full suck feeds. Multiple phototherapy sessions were administered for treatment of jaundice owing to large cephalhematoma. Cranial ultrasound confirmed the presence of cephalhematoma along the left parietal convexity and with small benign choroid plexus cysts on both right and left germinal matrices.
Empirical antibiotics were started on day 1st of life in view of sick clinical condition along with initial laboratory parameters including leucocytosis (WBC-29090/ cubic millilitres), and absolute neutrophilia (15796/ cubic millilitres), and thrombocytopenia (31000/ cubic millilitres), which were later stopped after negative septic screen and blood culture report. Subsequent hemogram showed leucocyte count of 25950/ cubic millilitres and platelet count of 32000/ cubic millilitres, haemoglobin was equivalent to previous lab report as 16.1 gram/dl. Peripheral smear disclosed macrocytic hypochromic picture with anisocytosis, polychromasia, nucleated RBCs with microspherocytes along with neutrophilia, monocytosis and thrombocytopenia. Platelets count improved to 118000/ cubic millilitres after a transfusion of single donor platelets in view of bleeding manifestations in the form of petechial rashes over the body and identification of small amount of blood in orogastric aspirate. Coagulation profile (Prothrombin time 10.6s, International normalized ratio 0.99 and activated partial thromboplastin time 49s) and liver functions (total bilirubin 3.3 mg/dl, serum glutamic pyruvic transaminase, 21.9 U/L, serum glutamic oxaloacetic acid 45.1 U/L) were within normal range. Repeat hemogram done after 4 days of transfusion because of appearance of fresh petechial rash over lower back identified thrombocytopenia (30000/cubic millilitres), with normal haemoglobin, WBC and neutrophil counts, and hence necessitated platelets transfusion. At this point, workup for alloimmune thrombocytopenia with TORCH profile for mother was planned after a paediatric haematologist consultation. Intravenous Immunoglobulin (IVIG) transfusion was advised considering possibility of immune mechanisms leading to thrombocytopenia. However, antibody and TORCH workup could not be done because of parental financial constraints. Baby’s TORCH profile was within normal range. Subsequent platelets count done after 48 hours improved to 41000/cubic millilitres. Baby was closely observed for next 2 days for any bleeding manifestation but no such manifestation was noticed. Follow up hemogram was suggested after a week, which showed normal values (Figure 1).
Figure 1: Line diagram showing trend of platelet counts and interventions taken

Infantogram, performed to see any skeletal anomaly including radial absence but it was normal. Karyotype sent confirmed trisomy 21 and concluded the diagnosis of Down’s syndrome. Ophthalmology examination revealed whitish opacity with bilateral immature cataract. Neonatal screening and hearing screening sent were normal.
Baby was discharged with normal hemodynamic and advised to visit to paediatric haematologist, developmental paediatrician, paediatric ophthalmologist including neonatologist. She is scheduled for cataract surgery by the age of six weeks and visual rehabilitation to prevent irreversible amblyopia and sensory nystagmus.
Discussion
This case describes the rare manifestations of thrombocytopenia and bilateral cataract in a newborn diagnosed with Down’s syndrome. The largest case series of babies documented with Down’s syndrome with hematological abnormalities including thrombocytopenia, published in 2019 by Shahin et al 12; showed that thrombocytopenia was identified as the second most common (66%) hematological abnormality after neutrophilia (80%). Karkurt et al conducted a large single centre study for thirteen years to review hematological disturbances in neonates with down’s syndrome and found that thrombocytopenia was a frequent finding of the study (26%). Thrombocytopenia was hypothesized to be occurring because of decreased production due to transient myelosuppression or increased destruction or both. 13
The reason for thrombocytopenia is largely unknown however temporary lack of regulation of the platelet precursor cells could be considered as one of the theories for the neonates with Down’s syndrome. Matsubara et al 14 studied the relationship of thrombopoietin (TPO) and platelet count in thrombocytopenic DS neonates and concluded that TPO in DS infants rose from birth to day 2, and thereafter gradually decreased by the end of the neonatal period.
The prevalence of cataract in DS ranges from 5-50% 7, and is mostly acquired, attributed to increased levels of superoxide dismutase 15 leading to elevated level of reactive oxygen species. Lenticular deposition of amyloid -Beta peptide is thought to be another factor contributing to development of cataract 16. Haargaard and Fredelius et al 7estimated a population-based frequency of 1.4% of early cataract in children aged 0-17 years however Rahl and Dezateux et al 11 reported a bilateral congenital or infantile cataract rate of 66% in a cohort of 243 patients and suggested that the rate of bilateral cataract may be higher in children with DS as compared to general paediatric population.
Regarding surgical outcome; Saifee et al has concluded that the rate of post operative complications in patients underwent cataract surgery with DS was comparable to that of general pediatric population, except for the increased risk of retinal detachment 19. In a cohort of 1043 eyes of 656 children underwent cataract surgery, Haargaard et al reported that 20 years risk of retinal detachment only 3% whereas it was eight times (23%) in children with mental sub normality including Down’s syndrome 20.
Conclusion
Karyotyping should be mandatory if there is dysmorphism to establish the diagnosis especially when it is associated with transient thrombocytopenia and cataract. This will further substantiate the need of extra cautious of bleeding tendencies and requirement of CBC and frequent peripheral smears with platelet transfusions. Frequent follow up visits for hematological concern should be mandatory, as these patients will often require some surgical interventions. Transient Myeloproliferative disorder could be another possibility if thrombocytopenia persisted beyond 8-12 weeks after birth in DS babies and it warrants long term follow up to see complications associated with it, like transient Leukemia. 17,18
Detailed ophthalmologic work up is of utmost important and if clinical condition permits cataract surgery also to be planned as early as possible at around 6 weeks, as to prevent from sensory visual deprivation.
References
1. Thuring W, Tonz O. Neonatale Thrombozytenwerte bei Kindern mit Down- Syndrom und anderen autosomalen Trisomien. Helv paediat Acta, 34: 545- 555, 1979.
2. Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with down syndrome: data from a multihospital healthcare system. Am J Med Genet A. 2007; 143A (1):42-50.
3. Hord JD, Gay JC, Whitlock JA. Thrombocytopenia in neonates with trisomy 21. Arch Pediatr Adolesc Med. 1995; 149(7):824-5.
4. Miller M, Cosgriff JM. Hematological abnormalities in newborn infants with Down syndrome. Am J Med Genet. 1983; 16(2):173-7.
5. Tahani Ali Bin A. Hematological manifestation in Down syndrome. Int J Biotech Bioeng. 2017; 6(3):171-5.
6. Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986; 108(5 Pt 1): 749-55.
7. Haargaard B and Fledelius HC. Down’s syndrome and early cataract. Br J Ophthalmol 2006; 90: 1024–1027.
8. Li EY, Chan TC, Lam NM, et al. Cataract surgery outcomes in adult patients with Down’s syndrome. Br J Ophthalmol 2014; 98: 1273–1276.
9. Postolache L. Abnormalities of the optic nerve in Down syndrome and associations with visual acuity. Front Neurol 2019; 10: 633.
10. Yonemoto Y, Morishita S, Fukumoto M, et al. Bilateral rhegmatogenous retinal detachment due to unusual retinal degeneration in Down syndrome: a case report. Medicine (Baltimore) 2018; 97: e10896.
11. Rahi JS and Dezateux C. Congenital and infantile cataract in the United Kingdom: underlying or associated factors. British Congenital Cataract Interest Group. Invest Ophthalmol Vis Sci 2000; 41: 2108–2114.
12. Mafinezhad S, Boskabadi H, Bayani G, Ehteshammanesh H. Thrombocytopenia during the First Week of Life among Neonates with Down Syndrome: Data from Multihospital Healthcare Systems. Iranian Journal of Neonatology. 2019 Jun: 10(2). DOI: 10.22038/ijn.2019.31995.1444.
13. Karakurt N, Uslu ?, Aygün C, Albayrak C. Hematological disturbances in Down syndrome: single centre experience of thirteen years and review of the literature. Turk J Pediatr. 2019;61(5):664-670. doi: 10.24953/turkjped.2019.05.004. PMID: 32104997.
14. Matsubara K, Nigami H, Yura K, Inoue T, Isome K, Fukaya T. Serum thrombopoietin level and thrombocytopenia during the neonatal period in infants with Down's syndrome. J Perinatol. 2010 Feb;30(2):98-102. doi: 10.1038/jp.2009.120. Epub 2009 Aug 13. PMID: 19675574.
15. Puri BK and Singh I. Prevalence of cataract in adult Down’s syndrome patients aged 28 to 83 years. Clin Pract Epidemiol Ment Health 2007; 3: 26.
16. Moncaster JA, Pineda R, Moir RD, et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS ONE 2010; 5: e10659.
17. Lin AE, Basson CT, Goldmuntz E, Magoulas PL, McDermott DA, McDonald-McGinn DM, et al. Adults with genetic syndromes and cardiovascular abnormalities: clinical history and management. Genet Med. 2008; 10(7):469-94.
18. Gamis AS, Smith FO. Transient myeloproliferative disorder in children with Down syndrome: clarity to this enigmatic disorder. Brit J Haematol. 2012; 159(3):277-87.
19. Saifee M, Kong L and Yen KG. Outcomes of cataract surgery in children with Down Syndrome. J Ophthalmic Vis Res 2017; 12: 243–244.
20. Haargaard B, Andersen EW, Oudin A, et al. Risk of retinal detachment after pediatric cataract surgery. Invest Ophthalmol Vis Sci 2014; 55: 2947–2951.
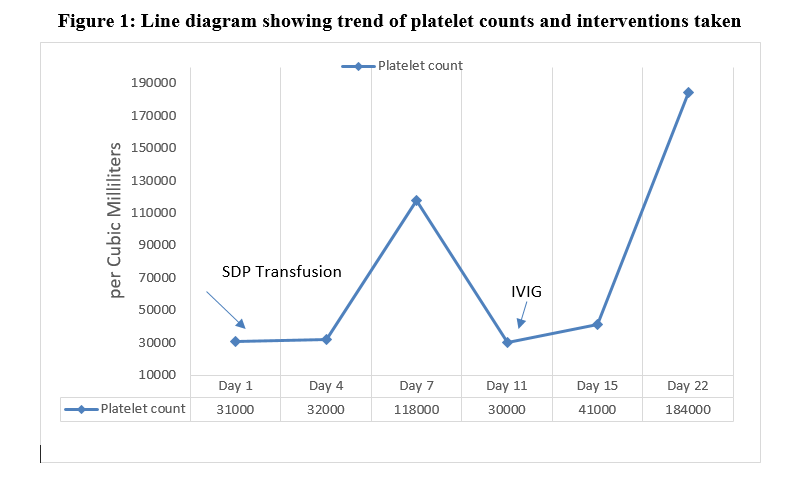
Figure 1
