Localization of the Parathyroid Gland and its Implications for Clinical Management
Localization of the Parathyroid Gland and its Implications for Clinical Management
Vinod Kumar Singhal 1*, Nufra Senopher2, Faris Dawood Alaswad3, Adil Mohammed Suleman4, Riya Singhal5
1. Vinod Kumar Singhal, Consultant surgeon, Department of General Surgery, Prime Hospital, Dubai, UAE
2. Nufra Senopher, Department of General Surgery, Prime Hospital, Dubai, UAE.
3. Faris Dawood Alaswad, Consultant General Surgeon, Department of Surgery, Gladstone Hospital, Perth, Australia.
4. Adil Mohammed Suleman, Specialist General Surgeon, Department of General Surgery, Prime Hospital, Dubai, UAE.
5. Riya Singhal, Student, SMCH, Dubai, UAE.
*Correspondence to Vinod Kumar Singhal, Consultant surgeon, Department of General Surgery, Prime Hospital, Dubai, UAE.
Copyright
© 2024 Vinod Kumar Singhal. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 October 2024
Published: 30 October 2024
DOI: https://doi.org/10.5281/zenodo.14160053
Abstract
Clinical Background: The incidence of hyperparathyroidism, especially primary hyperparathyroidism, has increased over the last decade due to improvements in diagnostic procedures and the increase in parathyroid surgery aimed at developing minor surgical planes. A prerequisite for targeted parathyroidectomy is prior surgery in the area of suspected parathyroid pathology. Therefore, parathyroid imaging modalities and protocols have multiplied, leading to confusion regarding their indications and applications.
Research: Data from clinical trials and reviews published since 2000 have been reviewed and supplemented with targeted studies using biomedical data repositories. We also benefit from our general diagnosis. Many variations exist, and many additional non-imaging techniques, such as computed tomography and magnetic resonance imaging, have been described. The good anatomical details of 4D CT should be balanced against the main radiation of the thyroid gland. Invasive intravenous PTH sampling and parathyroid arteriography have an important place in the management of cases. Multidisciplinary collaboration between endocrinologists, surgeons and radiologists is good because there is a lot of heterogeneity in the image. Conclusion: Parathyroid localization is suitable for surgical candidates. Important points to consider when selecting imaging studies include availability, cost, radiation exposure, local expertise and accuracy. Other important factors include the patient's pathology and whether the disease is relapsing or refractory. Imaging procedures are offered in patients with primary hyperparathyroidism.
Localization of the Parathyroid Gland and its Implications for Clinical Management
Case Study
Primary hyperparathyroidism (1°HPTH) is a common condition that affects the thyroid gland and can be diagnosed by endocrinologists. Imaging studies are often obtained to determine the disease etiology and direct operative planning. Early efforts at parathyroid localization were limited, but the benefits of focused parathyroidectomy, such as shorter operative times, lower incidence of post-operative hypocalcemia when unexplored parathyroids are left in situ, and decreased risk of recurrent laryngeal nerve injury, have encouraged the development of improved localization techniques. Parathyroid gland size, number, and location are some of the most variable features of human anatomy. The most typical arrangement is of two sets of paired glands adjacent to the posterior thyroid gland. The superior glands are derived from the fourth branchial pouch and are less common in patients with PTH hypersecretion from an adenoma due to negative feedback. Most glands are ovoid or bean-shaped, but elongated and multilobed glands are not uncommon. Supernumerary glands occur in up to 13% of patients.
The vast majority of parathyroid disease is benign and manifest by excess secretion of PTH, resulting in hypercalcemia. 1°HPTH is the most common disease of the parathyroids and most often results from a single adenoma consisting of a clonal population of proliferating cells. About 10-15% of 1°HPTH results from multigland disease (MGD) in the form of 4-gland hyperplasia or, less commonly, 2 independent (“double”) adenomas. Secondary hyperparathyroidism (2°HPTH) is a state of abnormal PTH secretion due to a known stimulus, while tertiary hyperparathyroidism (3°HPTH) implies the development of autonomous, unregulated PTH secretion.
Fig 1: The study uses sagittal and transverse ultrasounds to identify hypoechoic ovoid parathyroid adenomas in patients with a single adenoma. Sagittal ultrasounds show the adenoma is inferior and slightly posterior to the left lobe of the thyroid and the sternohyoid/sternothyroid muscles, while transverse ultrasounds show the adenoma posterior to the left thyroid lobe.
Surgery is the treatment of choice for symptomatic 1°HPTH, but some patients with 1°HPTH who meet specific criteria may be followed without surgery if certain recently reviewed guidelines are met. Medical therapy is the preferred initial treatment for 2°HPTH and 3°HPTH, but surgery is often required when medical treatment fails. In 1°HPTH, the operative approach is determined by etiology, with resection of the causative adenoma appropriate for single gland disease, 3.5-gland resection surgery used in most cases of MGD, and total parathyroidectomy with immediate heterotopic parathyroid autotransplantation. Bilateral cervical implantation (BCE) is a well-established approach with a cure rate in excess of 95% in the best centers.
It is crucial to consider parathyroid imaging as an adjunct to surgical therapy because imaging has no role in patients who are not surgical candidates. No localization procedure should be regarded as diagnostic, as aging has limited ability to differentiate between etiologies of parathyroid pathology and rarely contributes to therapeutic decision making. If the initial choice of operative approach is BCE due to the presence of concurrent thyroid pathology, high suspicion of MGD, surrogate preference, or other reasons, preoperative imaging is not required because all 4 parathyroid glands are usually identified intraoperatively.
Localization modality have proliferated, and studies examining their efficacy can be difficult to interpret due to heterogeneous definitions used to evaluate effectiveness. In almost all cases of “negative” imaging, an experienced endocrine surgeon will find disease during exploration. Therefore, any study evaluating parathyroid imaging that does not include correlation with operative findings and cure rates cannot by definition ascribe true positive or negative findings.
Ultrasound is a diagnostic tool used to examine parathyroid adenomas, which are homogeneous, hypoechoic structures that are ovoid and.8–1.5 cm in length. It was first described in the late 1970s and is performed using a high-resolution transducer with a frequency of 5–15 MHz. The patient is positioned supine with the neck extended, and Doppler images are obtained to identify ectopic glands. Enlarged parathyroids appear as homogeneous, hypoechoic structures that are ovoid and.8–1.5 cm in length. Cystic degeneration is present in 1–2% of enlarged glands, although very large adenomas may be irregularly shaped. Doppler images assist in distinguishing suspected parathyroid glands from alternative structures because parathyroid adenomas typically have a peripheral rim of vascularity and asymmetrically increased blood flow compared to the thyroid.
Ultrasound has an overall sensitivity of 70–80% in patients with a single adenoma, and specificity in excess of 90%. It is inexpensive and widely available, does not expose the patient to ionizing radiation, and has satisfactory sensitivity to be employed as a first-line imaging study. Furthermore, it permits concomitant evaluation for thyroid pathology, which occurs in 29–51% of 1°HPTH patients. Ultrasound also facilitates percutaneous biopsy, and preoperative sonography combined with fine-needle aspiration (FNA) of thyroid nodules can reduce the need for simultaneous thyroid surgery from 30% of 1°HPTH patients to 6% (30). However, sonography is limited by body habitus and gland morphology. Parathyroid gland size and volume are key predictors of ultrasound detection sensitivity by 10% and accuracy by 54% (22). Graded compression can improve visualization of small adenomas up to 27% of the time because adenomas are less compressible than surrounding tissue (23) and adjuctive techniques such as axial rotation of the patient’s head or swallowing may improve visualization of ectopic adenomas by shifting them into the sonographer’s view. Scintigraphy, another diagnostic tool, was first reported in 1983 and has since been extensively studied. The description of technetium 99m (99mTc)-sestamibi scintigraphy in 1989 greatly increased the utilization and sensitivity of nuclear imaging and remains the agent of choice. Sestamibi scintigraphy has several advantages, including being widely available, having less ambiguity or operator dependence in interpreting the results compared to sonography, being relatively inexpensive, and featuring a wide field of view to assess for ectopic glands.
Fig 2: 99mTc-sestamibi scintigraphy reveals left lower parathyroid adenoma, with physiological uptake in heart, thyroid, submandibular, and parotid glands. Sensitivity ranges from 54-96%, best with adjunctive SPECT techniques.
The clearest disadvantage of sestamibi scintigraphy is the potential for false-positive results. Both benign and malignant thyroid nodules can cause incorrect characterization of sestamibi nuclear imaging; a nodular thyroid reduces sensitivity by 15–39% (36, 37). Follicular and Humphrey cell thyroid neoplasms are particularly prone to sestamibi accumulation (38), and inflammationary thyroiditis and cervical lymphadenopathy are other common causes of false-positive scans. Overall, effectiveness of 99mTc-sestamibi imaging is strongly correlated with adenoma size, with gland weight of < 600–800 mg associated with false-negative results. Greater aberrations in serum PTH, calcium, and vitamin D levels have also been correlated with improved scan effectiveness.
A wide variety of sensitivities have been reported for 99mTc-sestamibi parathyroid scintigraphy, ranging from 54 to 96%. In a meta-analysis of 20 225 patients with 1°HPTH, Ruda et al found an overall sensitivity of 88% for detection of a single adenoma. Sensitivity decreased to 45 and 30% in patients found to have 4-gland hyperplasia or double adenomas, respectively.
Sestamibi scintigraphy is a versatile diagnostic technique used to detect and visualize parathyroid tumors. One of the most useful variants is 3-dimensional single-photon emission computed tomography (SPECT), which assists surgical exploration by demonstrating lesion location in all three dimensions and allows visualization of posterior adenomas in the retroesophageal position that is otherwise masked by thyroid tracer uptake. This results in improved sensitivity vs planar imaging, with a positive predictive value of 78.9 and 90.7% for sestamibi-SPECT.
Additional variants in sestamibi scintigraphy include dual-phase imaging, which adds an early image acquisition phase at 10-15 minutes after sestamibi administration in addition to the standard 90- to 180-minute window. This allows localization of tracer uptake in relation to the thyroid and can visualize the uncommon parathyroid adenoma that exhibits rapid sestamibi washout not visible on late phase images. Dual-phase imaging results in a small gain in sensitivity vs single-phase protocols, while subtraction imaging is performed by adding a second thyroid-specific radiopharmaceutical such as 99mTc-pertechnetate or 123Iodine to allow digital subtraction of the thyroid signal from 99mTc-sestamibi signal.
An emerging technique involves the combination of 99mTc-sestamibi SPECT scintigraphy and computed tomography (CT). Although the timing of the hybrid SPECT/CT protocol results in CTimages of less detail than conventional diagnostic CT, the improvement in anatomical detail over SPECT alone is notable. This results in improved differentiation between spurious sources of 99mTc-sestamibi uptake such as the thyroid or cervical lymph nodes and also provides improved anatomical formation for ectopically located parathyroid adenomas. A comprehensive study by Lavely et al (42) examined 110 patients with 1°HPTH who underwent dual-phase 99mTc-sestamibi with planar, SPECT, and SPECT/CT imaging. Early SPECT/CT in combination with any modality for delayed imaging emerged as the best overall protocol, with an accuracy of approximately 86%.
Positron emission tomography (PET) has also been evaluated in patients with 1°HPTH for preoperative localization. Fluorodeoxyglucose-PET detects increased glucose metabolism and has a reported sensitivity in 1°HPTH of 86%, but it has not been widely employed. A PET/CT hybrid technique using 11C-methionine has also been recently described. Sensitivity approximates standard 99mTc-sestamibi scintigraphy (approximately 83%), but has an increased sensitivity for MGD (67%).
Conventional CT for parathyroid localization has generally been inferior to other modalities, with a mean sensitivity of approximately 40-70%. However, recent developments have emerged, such as 4D-CT, which relies upon the perfusion characteristics of parathyroid adenomas and has been shown to be highly effective in cases of reoperative neck surgery. 4D-CT has been shown to be an effective primary localization study for all patients with 1°HPTH, correctly localizing the quadrant of the neck in 85.7% of cases.
Magnetic resonance (MR) has a reported sensitivity for abnormal parathyroid glands ranging from 43-71%. The chief advantages of MR are lack of radiation, detailed anatomical information, and the ability to widely evaluate ectopic glands. However, MR is largely limited to an adjunctive role during reoperative cases because it is costly and prone to motion artifact due to lengthy image acquisition times.
Catheter-based identification of abnormal parathyroid glands involves two distinct methodologies: selective venous sampling (SVS) and parathyroid arteriography. In recurrent or persistent hyperparathyroidism or patients who have undergone significant nonparathyroid cervical surgery, the benefit of parathyroid localization outweighs the risks of invasive techniques, such as catheter injury, hematoma, contrast-induced nephropathy, anaphylaxis, and stroke.
SVS is the "gold standard" invasive technique, typically obtained via the femoral vein. Serial blood samples are drawn from the superior vena cava and bilatary brachiocephalic, internal jugular, vertebral, thymic, superior, middle, and inferior thyroid veins. PTH is assayed in each sample, and the data are analyzed for a gradient indicative of parathyroid location. In-suite utilization of a rapid PTH assay improves response time and data fidelity, as well as facilitating additional sampling. Sensitivity of SVS in reoperative cases of patients with 1°HPTH ranges from 71–90%.
Parathyroid arteriography is both complementary and an adjunct to SVS, with contrast media infusion near the thyrocervical trunk demonstrating abnormal parathyroid tissue as a hypervascular blush and guiding operative exploration and confirm a grain suggested by SVS. Arteriography can be especially valuable in patients with prior neck surgery where the usual parathyroid venous drainage can be profoundly altered.
Image-guided confirmation of suspicious lesions has also been demonstrated using ultrasound- or CT-guided FNA. A PTH assay is performed on the FNA aspirate to determine whether the index lesion is a parathyroid gland. An on-site rapid PTH assay allows quick turnaround and rebiopsy if necessary for inconclusive results. Positive identification of a suspicious gland also implies a focused operative approach. Wire-guided explora-tions after CT- or ultrasound-guided localization have also been described.
References
1. Doppman JL. Reoperative parathyroid surgery; localization procedures. Prog Surg. 1986;18:117–132.
2. Udelsman R, Lin Z, Donovan P. The superiority of minimally invasive parathyroidectomy based on 1650 consecutive patients with primary hyperparathyroidism. Ann Surg. 2011;253:585–591.
3. Sackett WR, Barraclough B, Reeve TS, Delbridge LW. Worldwide trends in the surgical treatment of primary hyperparathyroidism in the era of minimally invasive parathyroidectomy. Arch Surg. 2002; 137:1055–1059.
4. Akerstro¨ m G, Malmaeus J, Bergstrom R. Surgical anatomy of hu- man parathyroid glands. Surgery. 1984;95:14 –21.
5. Grimelius L, Bondeson L. Histopathological diagnosis of parathyroid diseases. Pathol Res Pract. 1995;191:353–365.
6. Yao K, Singer FR, Roth SI, Sassoon A, Ye C, Giuliano AE. Weight of normal parathyroid glands in patients with parathyroid adeno- mas. J Clin Endocrinol Metab. 2004;89:3208 –3213.
7. Imanishi Y, Tahara H, Palanisamy N, et al. Clonal chromosomal defects in the molecular pathogenesis of refractory hyperpara thyroidism of uremia. J Am Soc Nephrol. 2002;13:1490 –1498.
8. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. be- tween 1985–1995: a National Cancer Data Base Report. The Amer- ican College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86:538 –544.
9. Bilezikian JP, Khan AA, Potts JT Jr. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary state ment from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–339.
10. Allendorf J, DiGorgi M, Spanknebel K, Inabnet W, Chabot J, LoGerfo P. 1112 Consecutive bilateral neck explorations for primary hyperparathyroidism. World J Surg. 2007;31:2075–2080.
11. Irvin GL III, Prudhomme DL, Deriso GT, Sfakianakis G, Chandarlapaty SK. A new approach to parathyroidectomy. Ann Surg. 1994; 219:574 –579.
12. Irvin GL III, Dembrow VD, Prudhomme DL. Operative monitoring of parathyroid gland hyperfunction. Am J Surg. 1991;162:299 – 302.
13. Udelsman R, Donovan PI, Sokoll LJ. One hundred consecutive minimally invasive parathyroid explorations. Ann Surg. 2000;232:331– 339.
14. Miccoli P, Berti P, Materazzi G, Massi M, Picone A, Minuto MN. Results of video-assisted parathyroidectomy: single institution’s six- year experience. World J Surg. 2004;28:1216 –1218.
15. Gagner M. Endoscopic subtotal parathyroidectomy in patients with primary hyperparathyroidism. Br J Surg. 1996;83:875.
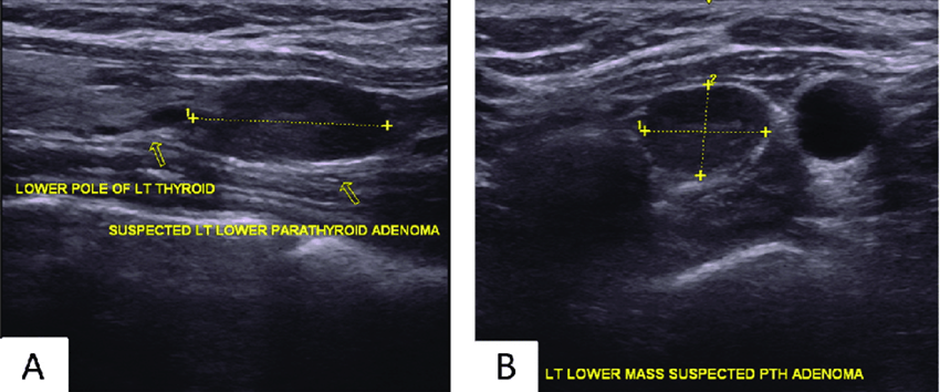
Figure 1
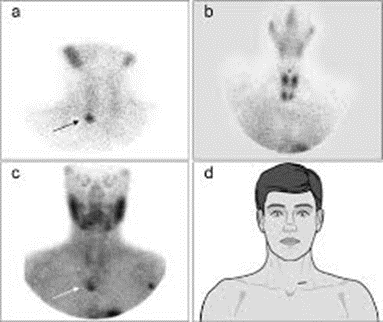
Figure 2
