Treatment of Infertility Using Platelet-Rich Plasma
Treatment of Infertility Using Platelet-Rich Plasma
Archana Singh1*, Varsha Ojha2
1) Archana Singh, Consultant IVF & Gynecologist, Indira IVF, Jodhpur, India.
2) Varsha Ojha, Specialist Gynecologist & Obstetrician, Prime Hospital, Dubai, UAE.
*Correspondence to: Archana Singh, Consultant IVF & Gynecologist, Indira IVF, Jodhpur, India.
Copyright
© 2024 Archana Singh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 22 October 2024
Published: 01 November 2024
DOI: https://doi.org/10.5281/zenodo.14160357
Treatment of Infertility Using Platelet-Rich Plasma
Introduction
Regenerative biomedicine is gaining popularity in various medical disciplines, with Platelet-rich plasma (PRP) becoming a popular nonoperative treatment option for various medical disorders. PRP is used in orthopedic and sports medicine to relieve pain and promote healing in musculoskeletal diseases like tendonitis, arthritis, ligament sprains, and tears. Researchers across various specialties have found growth factors to promote natural healing responses. PRP is an autologous concentration of platelets in a small volume of plasma, usually 5 to 10 times the normal level of the whole blood. As platelets are concentrated, so are all growth factors. PRP does not require FDA approval as it comes from the patient's own body and is processed in a specialized kit and centrifuge without red cells.
Fig 1
PRP composition and activation
-> Platelets contain high concentrations of cytokines and growth factors stored within α-granules. These growth factors include platelet-derived growth factor, insulin-like growth factor, vascular endothelial growth factor, platelet-derived angiogenic factor, transforming growth factor beta, fibroblast growth factor, epidermal growth factor, connective tissue growth factor, and interleukin-8. In addition to growth factors, platelets contain other substances, such as fibronectin, vitronectin, and sphingosine 1-phosphate, that initiate wound healing
-> Platelet activation triggers the release of these growth factors by a variety of substances or stimuli such as thrombin, calcium chloride, and collagen. Each method influences both the physical form of PRP and the amount of growth factors released, as well as the kinetics of release. No evidence has been found regarding the ideal concentration of activator required to trigger the optimal release of growth factors during the activation process of PRP, and different concentrations may therefore lead to different results
The theory behind this treatment method is based on scientific research. It was derived from natural healing processes, as the body's first response to tissue injury is to deliver platelets to the injured area. Platelets promote healing and attract stem cells to the site of the injury. Moving from basic science to clinical practice, PRP injections have been applied to diseased.
Fig 2, Fig 3, Fig 4
Various aspects of endometrial rejuvenation using Platelet-Rich Plasma (PRP) therapy, primarily in the context of fertility treatments. Let me elaborate on each section in a more detailed and organized way:
1. Endometrial Rejuvenation
Endometrial rejuvenation is aimed at improving the health and thickness of the uterine lining (endometrium), which is crucial for successful embryo implantation during fertility treatments like IVF or ICSI. This therapy is particularly beneficial in patients who suffer from conditions that result in a thin or damaged endometrium, which makes implantation and pregnancy less likely.
A. Asherman Syndrome (A Thin and Damaged Endometrium)
- Asherman syndrome is a condition characterized by the formation of scar tissue in the uterine cavity, leading to a thin and damaged endometrium. This condition often results from previous surgeries or infections.
- Treatment involves hysteroscopic adhesiolysis (removal of adhesions) followed by PRP infusion into the uterus. PRP, which contains growth factors, helps promote healing and regeneration of the endometrial lining.
- After the PRP infusion (0.5-1ml via catheter), estrogen therapy is used to support further regeneration. In more severe cases, 5ml of PRP may be injected directly into the uterine wall, followed by the insertion of a Foley’s balloon catheter, which is left for two weeks to keep the cavity open and aid in tissue recovery.
B. Endometrial Rejuvenation with IVF or ICSI (A Thin Endometrium)
- In some women undergoing IVF or ICSI, the endometrial thickness remains below the optimal level (< 7mm) despite hormone replacement therapy (HRT). Thin endometrium can lead to the cancellation of the embryo transfer cycle, reducing the chances of pregnancy.
- PRP therapy (0.5-1ml) is used in such cases, where it is infused into the uterine cavity on the 10th day of the HRT cycle. If there is no improvement in endometrial thickness after 72 hours, additional PRP infusions are performed.
- Once the endometrial thickness exceeds 7mm, embryo transfer is conducted. Studies have shown improved endometrial growth and pregnancy rates in women after PRP therapy.
Fig 5
C. PRP in Repeated Implantation Failure (A Normal Endometrium)
- Repeated Implantation Failure (RIF) refers to the inability to achieve pregnancy after several embryo transfers during IVF cycles. In cases of RIF, although the endometrium appears normal in thickness, other factors such as endometrial receptivity and immunological issues may hinder implantation.
- Intrauterine infusion of PRP, typically 0.5ml, is done 48 hours before embryo transfer to promote endometrial growth and improve receptivity. PRP's growth factors help create a more favorable environment for embryo implantation.
2. Ovarian Rejuvenation with PRP
- Ovarian rejuvenation using PRP is a promising therapy for women with diminished ovarian reserve or those experiencing perimenopause, menopause, or premature ovarian failure (POF). The goal is to stimulate the ovaries and improve their function.
- PRP injections (2-4ml) are administered directly into the ovaries. This procedure involves puncturing the ovary at multiple sites to distribute the PRP and enhance its regenerative effects.
- This process is minimally invasive, done under local anesthesia, and typically repeated 2-3 times. The results are promising, with patients showing improved ovarian function, follicle development, and oocyte quality, leading to successful IVF outcomes.
Fig 6
3. PRP in Ovarian Torsion
- PRP therapy has also been explored for its protective effects in cases of ovarian torsion, where the ovary twists around the ligaments that support it, cutting off its blood supply. This can lead to ischemia (lack of blood flow) and reperfusion injury once the blood supply is restored.
- In experimental settings, PRP has been shown to reduce oxidative stress and tissue damage when administered before detorsion (untwisting of the ovary), making it a potential therapeutic option to prevent long-term ovarian damage.
4. Azoospermia and PRP Therapy
- Azoospermia, the absence of sperm in semen, can be non-obstructive, meaning the testes do not produce enough sperm. In recent developments, PRP combined with mesenchymal stem cells has been used as a therapy.
- The stem cells, isolated from the patient's own fat tissue, are mixed with PRP and injected into the testicles. This combination aims to initiate and improve spermatogenesis (sperm production). While still in experimental phases, this approach offers hope for men with non-obstructive azoospermia.
After PRP Therapy, it is advised to avoid sexual activity, steam baths, sauna visits, and using cotton underwear for 5 days. To prevent hyperpigmentation at the injection site, it is not advised to use a tanning bed or tanning bed for 1 week.
Conclusion:
PRP therapy is an affordable, simple, and effective noninvasive treatment for fertility-related issues like thin endometrium, ovarian dysfunction, and azoospermia. It is a minimally invasive approach that can improve the success rates of fertility treatments like IVF and ICSI. However, large randomized controlled studies are needed to confirm its efficacy and safety in various gynecological disorders. PRP therapy is still evolving, but early results suggest its potential in restoring reproductive function for many patients who previously had limited options. Further research is needed to confirm its efficacy and safety in various gynecological disorders.
Reference
1. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
2.Schmitz JP, Hollinger JO. The biology of platelet-rich plasma. J Oral Maxillofac Surg. 2001;59:1119–1121. doi: 10.1053/joms.2001.26801. [DOI] [PubMed] [Google Scholar]
3.Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online. 2017;35:343–350. doi: 10.1016/j.rbmo.2017.04.007. [DOI] [PubMed] [Google Scholar]
4.Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Fox L, Klement GL, Folkman J. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010;85:487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
5.Dawood AS, Salem HA. Current clinical applications of platelet-rich plasma in various gynecological disorders: An appraisal of theory and practice. Clin Exp Reprod Med. 2018;45:67–74. doi: 10.5653/cerm.2018.45.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
6.Marques LF, Stessuk T, Camargo IC, Sabeh Junior N, dos Santos L, Ribeiro-Paes JT. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets. 2015;26:101–113. doi: 10.3109/09537104.2014.881991. [DOI] [PubMed] [Google Scholar]
7.Tesarik J, Junca AM, Hazout A, Aubriot FX, Nathan C, Cohen-Bacrie P, Dumont-Hassan M. Embryos with high implantation potential after intracytoplasmic sperm injection can be recognized by a simple, non-invasive examination of pronuclear morphology. Hum Reprod. 2000;15:1396–1399. doi: 10.1093/humrep/15.6.1396. [DOI] [PubMed] [Google Scholar]
8.Yu EJ, Ahn H, Lee JM, Jee BC, Kim SH. Fertilization and embryo quality of mature oocytes with specific morphological abnormalities. Clin Exp Reprod Med. 2015;42:156–162. doi: 10.5653/cerm.2015.42.4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
9.Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, Sapienza F, Baroni E, Litwicka K, Greco E. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–1700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
10.Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT Jr, Tiras B, Seli E. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY) 2020;12:10211–10222. doi: 10.18632/aging.103403. [DOI] [PMC free article] [PubMed] [Google Scholar]
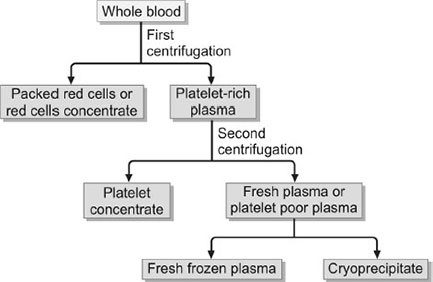
Figure 1
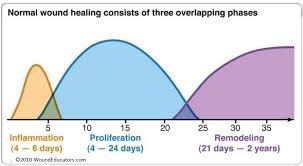
Figure 2
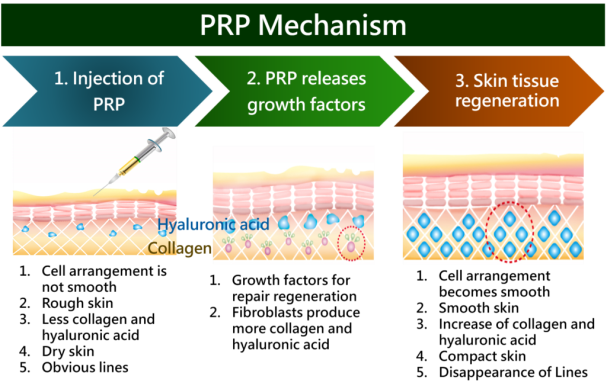
Figure 3
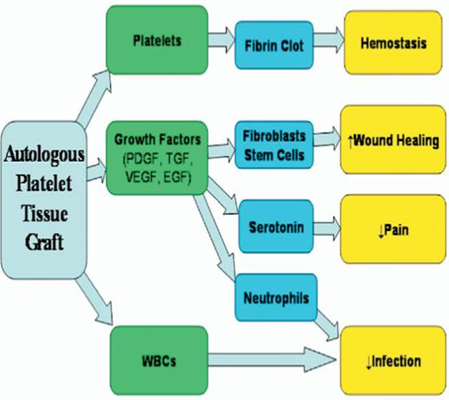
Figure 4
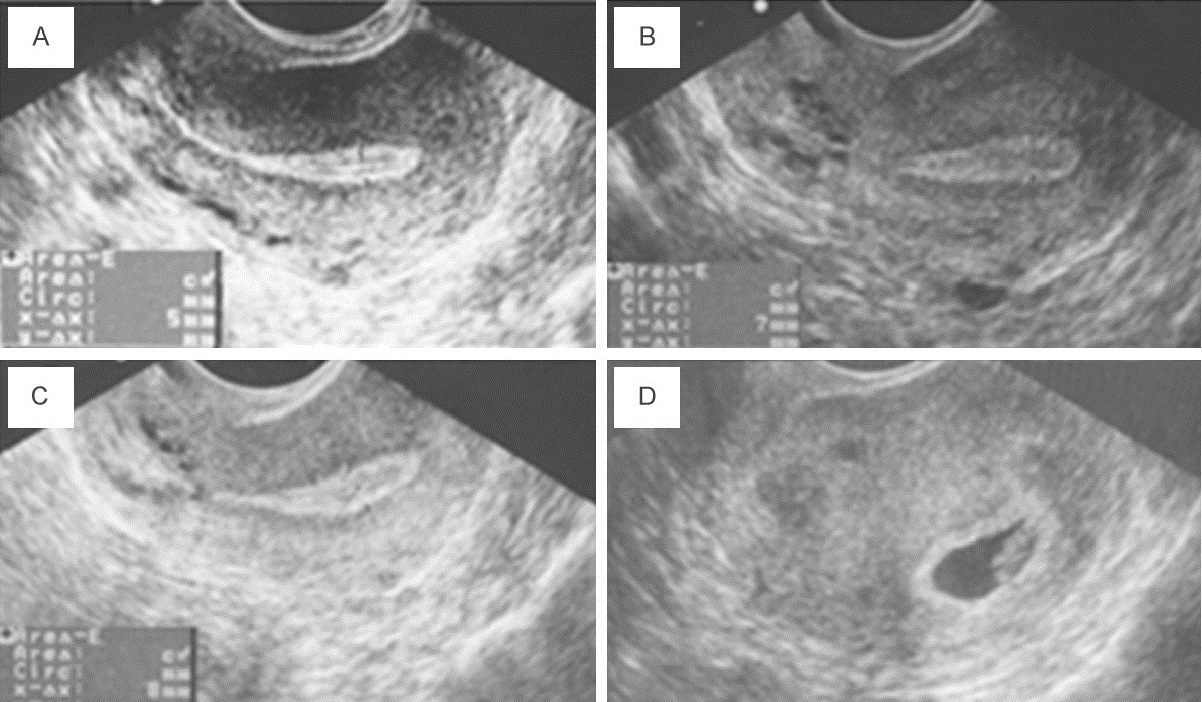
Figure 5
Figure 6
