Maxillofacial Management of Mandibular Malignant Lymphoma Case Report from Egypt.
Maxillofacial Management of Mandibular Malignant Lymphoma Case Report from Egypt.
Mohamed Said Hamed *1, Mohamed ElSholkamy*1, Merhan N. El-Mansy 2, Anas Taalab3, Eman M. Eldemardash3, Mona S. Shata 2, AlShaimaa Saeed4.
1. Professor of Oral and Maxillofacial Surgery, Faculty of Dentistry, Suez Canal University, Egypt.
2. Associate Professor of Oral and Maxillofacial Pathology, Faculty of Dentistry, Suez Canal University, Egypt.
3. Assistant lecturer of Oral and Maxillofacial Surgery, Faculty of Dentistry, Suez Canal University, Egypt.
4. Resident of Oral and Maxillofacial Surgery, Faculty of Dentistry, Suez Canal University, Egypt.
*Correspondence to: Mohamed Said Hamed, Professor of Oral and Maxillofacial Surgery, Faculty of Dentistry, Suez Canal University, Egypt.
Copyright
© 2024: Mohamed Said Hamed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 20 Sept 2024
Published: 06 Nov 2024
Abstract
Introduction: Lymphoma is a malignant disease of the lymphatic system. There are two types of lymphoma: Hodgkin's and non-Hodgkin's. They behave, grow, and respond to treatment differently. Diffuse large B-cell lymphoma (DLBCL) is a common type of fast growing (high grade) NHL.
Subjects and Methods: A 65-year-old male patient with mild age-related hypertension complained of a painful mass related to the lower right posterior area that started to develop after the extraction of a mobile lower right first molar over a month. It is easily bleeding and rapidly increasing in size. An incisional biopsy was performed for histological and immunohistochemical assessment.
Results: Histopathology and immunohistochemistry were consistent with DLBCL. H & E stained sections showed diffuse infiltration of large cells of varied sizes with nuclear pleomorphism and dilated blood vessels. There was strong immunopositivity with CD45, CD20 of the tumor cells and marked immune-negativity with CD3.
Conclusions: Histopathological analysis is necessary. Pathologists’ awareness of immunohistochemical biomarkers is important particularly in poorly differentiated neoplasms that help in differentiation between them. The early diagnosis can help the oncologists to choose the most suitable therapeutic strategy.
Key words: Oral Cancer, lymphoma, mandible, CD45, CD20, CD3.
Maxillofacial Management of Mandibular Malignant Lymphoma Case Report from Egypt.
Introduction
Lymphoma is a heterogeneous malignant disease of the lymphatic system. Based on statistics by the World Health Organization (WHO), lymphomas are the second most common malignancy in the oral cavity after squamous cell carcinoma. It is classified as Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (NHL).[i]
Non-Hodgkin’s lymphoma (97%) accounts for the most diagnosed type of all lymphomas.[ii] It usually has a more aggressive nature and is discovered at advanced stages due to its less predictable spreading patterns.[iii] NHLs are commonly present in nodal or extra nodal locations, including skin, stomach, and brain sites, and may spread unexpectedly. In the oral cavity, it is mainly presented in the gingiva, palatal mucosa then the mandibular bone. It is commonly occurring at the 7th decade of life, with male: female predilection of 1.5:1.[iv]
The lymphoma develops due to somatic mutation of a lymphocyte progenitor that leads to the proliferation of lymphocytic clones or their cell precursors or lymphocyte cell lines.[v] It is divided by immunophenotypic data into B-cell and T-cell lineages. Diffuse large B?cell lymphoma (DLBCL) being the most frequently diagnosed type of NHL in the oral cavity thar require immediate intervention due to its frequent growth of lymphadenopathy.[vi]
In this work, a biopsy should be taken for definitive diagnosis with performing immunological studies to provide an accurate treatment plan. It could be misdiagnosed with pyogenic granuloma, osteomyelitis, Ewing’s sarcoma, or squamous cell carcinoma.
Case Report
A 65-year-old male patient presented to the oral and maxillofacial outpatient clinic at the Faculty of Dentistry Teaching Hospital, Suez Canal University, Egypt. The medical history of the patient was mild age-related hypertension. The patient complained of a painful mass that spontaneously bleeds in the lower right posterior area. It started to develop and increase in size rapidly after the extraction of a mobile lower right first molar over a month period. The patient stated that, one month prior to the extraction, he felt numbness to the right side of the lower lip followed by severe pain in the lower right posterior area that was misdiagnosed as pulpitis in lower first molar then mobility was noticed.
Clinical examination: Extraoral examination showed facial asymmetry and swelling of the right side. The palpation of both submandibular and cervical lymph nodes did not show any abnormality. Intraoral examination showed heterogenous soft tissue mass extending from the lower right canine to the lower right second molar (figure 1). There was creeping on both buccal and lingual gingiva.
Radiographic investigations: The panoramic radiograph and cone beam computed tomography both revealed an ill-defined radiolucency extending from the distal aspect of lower right canine to the distal aspect of lower right second molar involving the inferior alveolar canal (IAC) (figure 2).
Laboratory investigation: The patient was asked to perform complete blood count (CBC) and bleeding profile before any surgical intervention. Their results came out normal except for mild normocytic normochromic anemia for age and sex. Leucocytic and platelets count was within normal range (figure 3).
Figure 1: (A) showing facial asymmetry and swelling of the right side. (B) showing intraoral soft tissue mass extending from the lower right canine to the lower right second molar
Figure 3: showing mild normocytic normochromic anemia for age and sex with normal count of leucocytes and platelets.
Incisional biopsy
Since the lesion size was large and showed different morphological characteristics, the decision to perform an incisional biopsy was necessary (figure 4). It was made from three different sites of mass to include all underlying histological features. During the surgery, the lesion was highly vascular that led to excessive bleeding. During the process of suturing, the tissue was friable and torn easily.
Figure 4: The planned three areas to be taken as samples in the incisional biopsy.
Histopathological investigations
The Histopathological H&E-stained section showed diffuse infiltration of large atypical lymphoid cells at various stages of differentiation, with cellular and nuclear pleomorphism. Nuclear hyperchromatism was a prominent feature. Scattered macrophages with single or two small and darkly stained nuclei were seen. The whole section was highly vascularized with widely dilated engorged blood vessels (Figure 5 A&B).
Immunohistochemistry was performed using standard techniques. Monoclonal antibodies were used for the following immunohistochemical markers: CD45 was used to differentiate hematological and non-hematological tumor cells. CD3 is a selective marker that indicates the presence of T-cells. CD20 is a selective marker that recognizes a subpopulation of B-cells. Strong immunopositivity was detected with cytoplasmic & membranous expression of CD45. Immuno-negativity of CD3 was markedly observed. Strong nuclear expression with CD20-positive cells was noticed (Figure 5 C: E). Both histopathology and immunohistochemistry were consistent with the diagnosis of Diffuse large B?cell lymphoma (DLBCL).
Figure 5: Diffuse large B cell lymphoma (A, B) diffuse infiltration of large cells of different sizes with scanty cytoplasm, nuclear pleomorphism, and prominent dilated & engorged blood vessels (A H&E x 20, B H&E x40). Immunohistochemistry shows (C) Cytoplasmic & membranous expression of CD45, (D) scanty cytoplasmic CD3 expression (E) strong CD20-positive cells (C&E IHC x40) , and (D IHC x 20).
Figure 6: PET scan shows mmultiple areas of isotope uptake denoting bone metastasis at the cervical & dorsal vertebrae, multiple ribs, right iliac bone, and the shaft of the left femur,
Referral to the oncology department:
A PET scan CT was performed, and combination of both radiotherapy & chemotherapy was initiated. (Figure 6) . After the first radiotherapy session, the patient showed signs of improvement.
Discussion
Diffuse large B cell lymphoma is considered one of the high-grade lymphomas, with 5 to 10-year survival (50-70%) of cases.[vii] The oral lymphoma usually presents as a painless mass that gradually increases in its size that may have superficial ulcerations.[viii] The incisional biopsy was extremely necessary for achieving definitive diagnosis and determining the best treatment plan for the patient. However, the lesion exhibited an accelerated growth pattern after the surgical intervention and within one week it was double sized. Therefore, the radiotherapy needed to be initiated once a diagnosis was made and even before the results of the special immunohistochemical stains.
The main treatment of the oral lymphoma is radiotherapy thus the communication with the oncology team needs to be as soon as possible.[ix] The age of the patient affects the prognostic factor. Older patients have a worse outcome than the corresponding younger patients. An immunological deterioration caused by the aging process may explain the occurrence of DLBCL without predisposed immunological disorders. (Takeshi Okamura, 2022)[x]
The histopathological features refer to diffuse large B-cell lymphoma (DLBCL). There was diffuse infiltration of large lymphatic cells with a large nucleus, prominent cellular & nuclear pleomorphism, and nuclear hyperchromatism. The large size of lymphoma cells is determined when they are larger than the nuclei of benign histiocytes.[xi] The updated 2016 classification of DLBCL was performed to help in diagnosis through detecting the cell of origin i.e., germinal center B?cell (GCB) versus activated B?cell or non?GCB type.[xii]
Further immunohistochemistry showed strong expression with CD45 & CD20 and negative expression with CD3. Leukocyte common antigen (CD45) is a transmembrane protein tyrosine phosphatase. It is located mostly on hematopoietic cells. The immunoreactivity of CD45 is thoughtful for non-Hodgkin’s lymphoma. It is used to differentiate between hematological & non-hematological malignancies.[xiii] Membranous and cytoplasmic reactions of different biomarkers in this case were compatible with similar reports. Specific T cell marker (CD3) is a common marker to detect absence or presence of T cells or natural killer cells. A pan B cell marker (CD20) is widely used to detect the type of infiltration of B lineage lymphoma cells. The staining pattern is considered as confirmatory method of such infiltration.[xiv] CD 20 is expressed from the naive B?cell until the last stages of B?cell development. Extensive CD20 positivity in the cytoplasm of tumor cells was suggestive of non-Hodgkin’s B cell lymphoma.[xv] Weak cytoplasmic expression of CD3 through the patient biopsy ensures the absence of T-cells in the tumor stroma, previous studies ensured the present results.[xvi]’[xvii]
Conclusion
Diffuse large B?cell lymphoma (DLBCL) being the most frequently diagnosed type of NHL in the oral cavity that require immediate intervention. Incisional biopsy and pathologists’ awareness of immunohistochemical biomarkers is important particularly in poorly differentiated neoplasms that help in diagnosis and differentiation between them. The early diagnosis of DLBCL can help the oncologists to choose the most suitable therapeutic strategy which can have a direct impact on the survival of the patient.
References
[i] Van der Waal, R. I. F., Huijgens, P. C., Van der Valk, P., & Van der Waal, I. (2005). Characteristics of 40 primary extranodal non-Hodgkin lymphomas of the oral cavity in perspective of the new WHO classification and the International Prognostic Index. International journal of oral and maxillofacial surgery, 34(4), 391-395.
[ii] El-Naggar, A. K., Chan, J. K., Rubin Grandis, J., & Slootweg, P. J. (2017). WHO classification of head and neck tumours. 4th ed. Lyon: International Agency for Research on Cancer.
[iii] Guevara-Canales, J. O., Morales-Vadillo, R., de Faria, P. E., Sacsaquispe-Contreras, S. J., Leite, F. P., & Chaves, M. G. (2011). Systematic review of lymphoma in oral cavity and maxillofacial region. Acta Odontológica Latinoamericana, 24(3), 245-251.
[iv] Reddy, I., Sreenath, G., Reddy, R., & Swathi, T. R. (2014). Non-Hodgkin’s lymphoma in buccal vestibule-case report. Journal of Clinical and Diagnostic Research: JCDR, 8(8), QD01.
[v] Mawardi, H., Cutler, C., & Treister, N. (2009). Medical management update: Non-Hodgkin lymphoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 107(1), e19-e33.
[vi] Bhattacharyya, I., Chehal, H. K., Cohen, D. M., & Al-Quran, S. Z. (2010). Primary diffuse large B-cell lymphoma of the oral cavity: germinal center classification. Head and neck pathology, 4, 181-191.
[vii] Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. (2001) WHO classification of tumour. Pathology and genetics of tumors of haematopoietic and lymphoid system. Ryon: IARC Press.
[viii] Takeshi Okamura, Toshiyuki Izumo, Ayataka Ishikawa, Yu Nishimura, Nobuko Kubota, Kazuhiro Yagihara, (2022). Malignant lymphoma of the oral cavity: A single-center study of 28 cases, Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology, 34)6, 698-703.
[ix] Gaweda, A., Jach, E., Wojciechowicz, J., Sokolowska, B., & Tomaszewski, T. (2014). Diffuse large B cell lymphoma of the oral cavity-case report. Journal of Pre-Clinical and Clinical Research, 8(1).
[x] Gutierrez, A., Mestre, F., Perez-Manga, G., & Rodríguez, J. (2011). Diffuse large B-cell lymphoma in the older. Critical reviews in oncology/hematology, 78(1), 59-72.
[xi] Li, S., Young, K. H., & Medeiros, L. J. (2018). Diffuse large B-cell lymphoma. Pathology, 50(1), 74-87.
[xii] Swerdlow, S. H., Campo, E., Pileri, S. A., Harris, N. L., Stein, H., Siebert, R., ... & Jaffe, E. S. (2016). The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, The Journal of the American Society of Hematology, 127(20), 2375-2390.
[xiii] Ngo, N., Patel, K., Isaacson, P. G., & Naresh, K. N. (2007). Leucocyte common antigen (CD45) and CD5 positivity in an “undifferentiated” carcinoma: a potential diagnostic pitfall. Journal of clinical pathology, 60(8), 936-938.
[xiv] Sabattini, E., Bacci, F., Sagramoso, C., & Pileri, S. A. (2010). WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica, 102(3), 83-87.
[xv]Shariatifar, H., Hakhamaneshi, M. S., Abolhasani, M., Ahmadi, F. H., Roshani, D., Nikkhoo, B., ... & Ahmadvand, D. (2019). Immunofluorescent labeling of CD20 tumor marker with quantum dots for rapid and quantitative detection of diffuse large B?cell non?Hodgkin's lymphoma. Journal of Cellular Biochemistry, 120(3), 4564-4572.
[xvi] Sethi, A., Tandon, A., Mishra, H., & Singh, I. (2019). Diffuse large B-cell lymphoma: An immunohistochemical approach to diagnosis. Journal of Oral and Maxillofacial Pathology, 23(2), 284-288.
[xvii] Saleh, M. M., Darwish, Z. E. S., Bahey, E., & Gaber, N. (2024). A CHALLENGING CASE REPORT OF DIFFUSE LARGE B-CELL LYMPHOMA. Advanced Dental Journal, 6(2), 203-207.
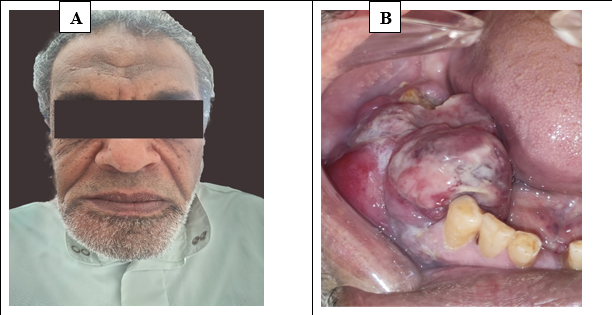
Figure 1
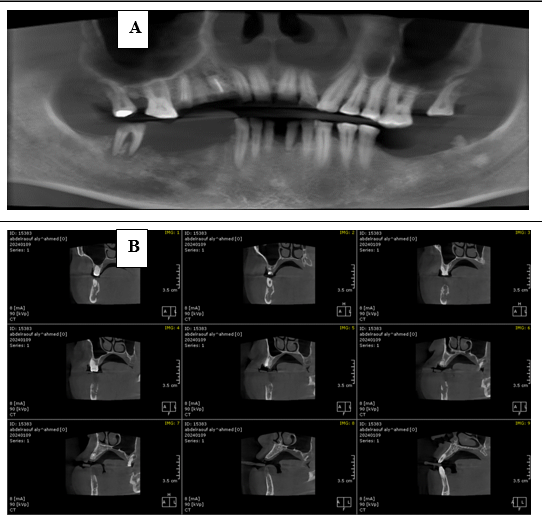
Figure 2
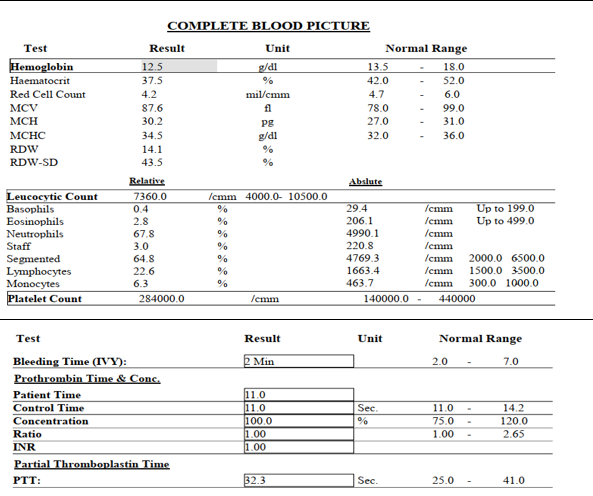
Figure 3
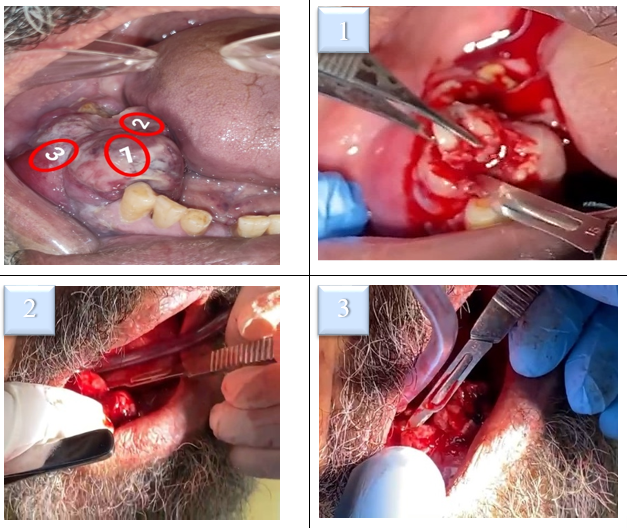
Figure 4
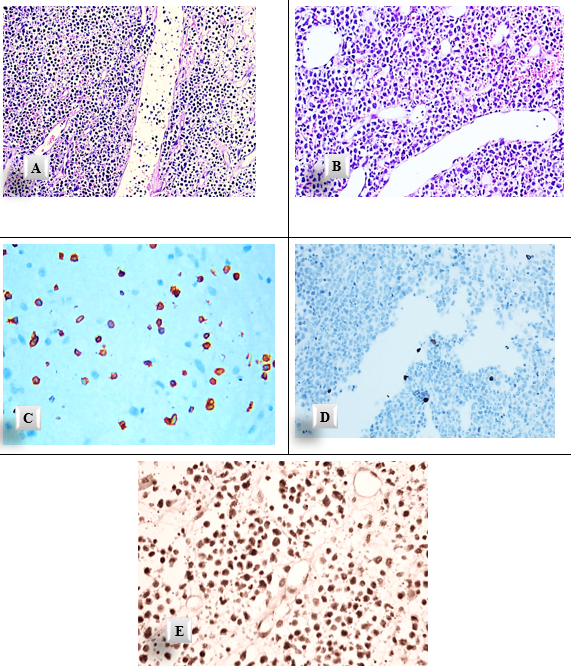
Figure 5
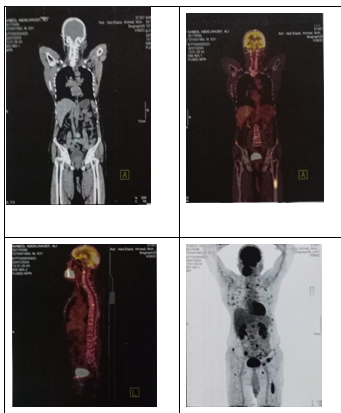
Figure 6
