Simple and Complex Anal Fistulas: Indications for Treatment in a General Surgery Department
Simple and Complex Anal Fistulas: Indications for Treatment in a General Surgery Department
Giovanni Salamina, MD*, Roberto Mario Lauro, MD, FACS, FRCS1, David Alessio Merlini, MD, EBSQ2
1,2. Department of General Surgery – G. Salvini Hospital- ASST Rhodense ( MI ) – Italy.
*Correspondence to: Giovanni Salamina- Via U. Pellegrini, 5, 20017 Rho, Milan, Italy.
Copyright
© 2024 Giovanni Salamina. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 01 November 2024
Published: 13 November 2024
DOI: https://doi.org/10.5281/zenodo.14126925
Abstract
In this paper, we selected a series of 80 consecutive patients from 2001 to 2019 suffering from simple and complex anal fistulas treated with fistulotomy with a combined loose and cutting seton technique.
We considered the rates of healing, recurrence and postoperative incontinence. The results are satisfactory and comparable to those of other series of dedicated coloproctologists.
We aim to demonstrate, mainly by using clinical and objective examination under anaesthesia, directly at the time of surgery, as even in a Department of General Surgery not-dedicated to Colo-rectal Surgery, such as ours, particularly complex anal fistulas can be treated with results comparable to those of Specialized Units.
Keywords: Anal, Fistula- in-ano, Seton, Surgery for transphincteric fistula.
Simple and Complex Anal Fistulas: Indications for Treatment in a General Surgery Department
Introduction
Anal fistulas, especially complex ones, have always represented a problem with surgical treatment, which is not univocal, depending on the different schools.
The literature lists techniques that are often very different, including the damblé fistulotomy for simple fistulas (1-4). For complex fistulas, various treatments are listed, including loose seton (5) rather than cutting seton (6); treatment with fibrin glue (7); LIFT (8) which involves the closure of the fistula in the space between the external and internal sphincter and the removal of the infected external tissue; fistulectomy plus flap (9) which consists of the removal of the fistula and the advancement of a full-thickness mucosal rectal flap to close the internal opening of the fistula; in more recent times, VAAFT (10), which consists in the closure of the internal orifice associated with cauterization of the fistula with video-assisted fistuloscopy; treatment with anal plug (11); FiLaC, which consists in laser cauterization of the fistulous tract (12).
Materials and Methods
We selected our case series of 80 consecutive anal fistulas from 2001 to 2019, operated by two surgeons of the same Operating Unit, of which 70% were simple and 30% complex. The patients were 30-80 years old, of which 50 were males and 30 were females.
The simple fistulas were intersphincteric and low transsphincteric (involving less than 30% of the sphincter system), and the complex fistulas (13-15) were trans-sphincteric involving more than 30% of the sphincter, recurrent fistulas, fistulas with multiple ramifications, fistulas with an anterior tract in women. Some complex fistulas are excluded from this case series, including horseshoe fistulas, in Crohn's disease and the rarer supra-sphincteric fistulas in which the tract passes through the entire external sphincter and part or all of the puborectalis muscle.
The diagnosis was performed with a proctological examination plus anoscopy for simple fistulas and for the majority of complex fistulas. It was completed in the operating room with an examination under anaesthesia and then speculation of the fistula after possible infiltration of hydrogen peroxide to identify the fistulous tract.
MRI was performed only for some complex fistulas with a not-identified tract. The treatment consisted of the removal of the extra-sphincteric portion of the fistula and positioning of a loose, not-cutting seton at the beginning and then tractioned on an outpatient basis, on average every 7-15 days, for medium-high but even low fistulas. In most cases, we preferred to position a seton also for intersphincteric fistulas, as a precaution, since these were essentially empirical diagnoses (because we did not investigate the fistulas preoperatively with endoanal ultrasound, which we did not have available in the short term).
We re-evaluated the patients in the outpatient clinic and established when to start pulling the seton, also in relation to the possible presence and persistence of an ongoing infection, while waiting for the possible regression thanks to drainage by the seton itself.
The surgical wound, when deep, was packed with swabs in the operating room and then checked twice a week in the outpatient clinic. The pack was generally removed after a few days with the improvement of the local inflammation and the appearance of scar granulation tissue.
The surgical wounds were washed with hydrogen peroxide and betadine solutions. The seton traction was performed in the outpatient clinic always from the inside of the seton according to the French school technique (16), positioning a tight silk thread near the sphincter, checking, with rectal exploration, the tone of the sphincter as well as the regression of the inflammation whose persistence represented the contraindication to the traction of the seton itself since it can be an expression of incorrect drainage of the fistula.
With this technique, the seton becomes progressively superficial until it falls spontaneously or through the surgeon's intervention with a section of the thin bridge of tissue (normally cutaneous) when it becomes extra-sphincteric.
Results
We lost 5 of the 80 treated patients to follow-up, 10 with simple fistulotomy and 70 with seton fistulotomy, with an average follow-up of 6 months. Of the remaining 75 patients, 67 recovered (89.3% recovery rate).
Therefore, we had a recurrence in 8 patients (11.7% of cases) which had, at least at the beginning, the persistence of local suppuration reappearance through the fistula.
We had mild incontinence, gas and rarely soiling, in 5 patients (6.6% of cases).
Five of the patients with recurrence were re-operated once and then recovered, and three twice. Of the latter, two recovered, and one was sent to another hospital at the request of the patient whose name we lost track of.
All patients with incontinence (subjective and confirmed by the score) (17) were re-evaluated in the outpatient clinic. All were recommended, at least initially, an astringent diet often with improvement of symptoms and signs. They were sent to another hospital with the indication to undergo biofeedback . A patient was even sent to another hospital for treatment with stem cells implantation (18) whose name we lost track of.
Conclusions and Discussion
Our results are consistent with those in the literature.
Perianal abscesses and fistulas are different aspects of the same pathology, which occurs in an acute form in the first case and represents the chronicity of the abscess in the second. In most cases, the cause is an infection of the anal glands (crypto glandular) (FIGURE 1).
Treatment of abscess is incision and drainage (evidence C TABLE 1-2).
Figure 1
TABLE 1 Grading of recommendations
|
Grade A Strong |
Recommendations supported by two or more level I trials without conflicting evidence from other level I trials |
|
Grade B Moderate |
Recommendations based on evidence from a single level I trial OR recommendations based on evidence from two or more level I trials with conflicting evidence from other level I trials OR supported by evidence from two or more level II trials |
|
Grade C Weak
|
Recommendations based on level III–V evidence |
Table 2: Levels of evidence
|
Level I |
Strong |
RCTs with p <0.05, inadequate sample sizes and/or inappropriate methodology |
|
Level II |
Moderate |
RCTs with p > 0.05, inadequate sample sizes and/or inappropriate methodology |
|
Level III |
Fair |
Non-randomized trials with contemporaneous controls |
|
Level IV |
Limited |
Non-randomized trials with historical controls |
|
Level V |
Consensus based |
Case series |
Perianal abscess, the antechamber of the fistula in 50% of cases, rarely heals spontaneously and often requires further surgical treatment following incision and drainage.
The goal of treating fistulas is to locate the internal orifice. From the moment of diagnosis of perianal abscess, it is mandatory to search for the possible internal orifice of a fistula. If the internal orifice is found, a seton is inserted, and it allows drainage of the abscess and possibly, at the same time, definitive treatment with fistulotomy.
In the search for the internal orifice, care must be taken not to create false paths and insist the probe can be responsible for an iatrogenic fistula.
Endoanal ultrasound, which is a tool routinely used in centres dedicated to proctology, possibly associated with the instillation of hydrogen peroxide through the external orifice of the fistula, if it is detectable, is the essential tool for evaluating whether a fistula has healed completely or whether there is still a path remaining.
Recurrence is usually due to incomplete drainage. Fistulotomy, if the path and the internal orifice of the fistula are identified with certainty, is performed for simple anal fistulas and leads to healing in 90% of cases (1-4). According to the guidelines in the literature, it can be performed damblé in lower trans sphincteric fistulas (19). We have almost always preferred to place a seton since the diagnosis was essentially made in the operating room with the patient in the gynaecological position subjected to subarachnoid anaesthesia, not being able to access an ultrasound scanner or even an MRI easily.
The patient was admitted to the day hospital, operated on, discharged, and subsequently followed in the outpatient clinic, where he will be subjected to dressings and washing with hydrogen peroxide and betadine solution and periodically seton traction.
Recurrence, which commonly occurs between 6-8 weeks and six months after surgery, therefore, also depends on the length of follow-up (20).
References
1. Cox SW, Senagore AJ, Luchtefeld MA, Mazier WP (1997) Outcome after incision and drainage with fistulotomy for ischiorectal abscess. Am Surg 63:686-89
2. Davies M, Harris D, Lohana P et al (2008) The surgical management of fistula- in- ano in a specialist colorectal unit. Int J Colorectal Dis 23:833-38
3. Ho KS, Tsang C, Seow- Choen F, Tang CL, Heah SM, Eu KW (2001) Prospective randomized trial comparing ayurvedic cutting seton and fistulotomy for low fistula-in-ano. Tech Coloproctol 5:137-41
4. Westertep M, Volkers NA, Poolman RW, van Tets WF (2003) Anal fistulotomy between Scylla and Charybdis. Colorectal Dis 5:549-51
5. Lentner A, Wienert V (1996) Long term, indwelling setons for low transsphincteric anal fistulas. Experience with 108 cases. Dis Colon Rectum 39:1097-1101
6. Khamar J, Sachdeva A, Mckechnie T, Lee Y, Tessier L, Hong D, Eskicioglu C (2023) Cutting seton for the treatment of cryptoglandular fistula–in- a: a systematic review and meta-analysis . Tech Coloproctol . PMID: 38091125 Review.
7. Lindsey I, Smilgin- Humphreys MM, Cunningham C, Mortensen NJ, George BD (2002). A randomized controlled trial of fibrin glue vs. conventional treatment for anal fistula. Dis Colon Rectum 45: 1608-15
8. A Rojanasakul (2009) LIFT procedure: a simplified technique for fistula-in-ano. Tech Coloproctol 13: 237-240
9. Balciscueta Z et al. (2017) Rectal advancement flap for the treatment of complex cryptoglandular anal fistulas: a systematic review and meta-analysis. Int J Colorectal Dis May 32 (5): 599-609
10. Meinero P, Mori L (2011) Video-assisted anal fistula treatment (VAAFT): a novel sphincter saving procedure for treating complex anal fistulas. Tech Coloproctol 15: 417-22
11. Garg P, Song J, Bhatia A, Kalia H, Menon GR (2010) The efficacy of anal fistula plug in fistula-in-ano: a systematic review. Colorectal Dis 12(10): 965-70
12. Wolicki A, Jager P, Deska T, Senkal M (2021) Sphincter- saving therapy for fistula- in –ano: long term follow- up after FiLaC
13. Amato A, Bottini C, De Nardi P, Giamundo P, Lauretta A, Realis Luc A, Piloni V. (2020). Evaluation and management of perianal abscess and anal fistu.la: SICCR position statement. Tech Coloproctol 24(2): 127-43
14. Parks AG, Gordon PH, Hardcastle JD (1976) A classification of fistula- in- ano. Br J Surg 63:1-12
15. Fazio VW (1987) Complex anal fistulae. Gastroenterol Clin North Am 16:93-114
16. Giordano M (2017) Case History Review of 2467 Anal fistulae Surgically Treated with the Method of Arnous’s French School Surg Technol Int (25) 30: 117-23
17. Jorge JM, Wexner SD (1993) Etiology And management of fecal incontinence. Dis Colon Rectum (36) 1: 77-97
18. Fernando del la Portilla et al. (2021) Treatment of fecal incontinence with autologous expanded mesenchimal stem cells: results of a pilot study. Colorectal Dis. 23 (3):698-709
19. Litta F, Prello A, Ferri L, Torrecilla NO, Marra AA, Orefice R, et al. (2021) Simple fistula- in-ano : is it all simple? A systematic revew. Tech. Coloproctol. 25(4):385-99
20. Zubing M, Qingming W,Yi Zhang, Peng L, Maojun Ge, Peixin Du, Wei Yang, Yazhou He (2019) Risk Factors for Recurrence after fistula surgery: A meta-analysis. Int J Surg. 69:153-64
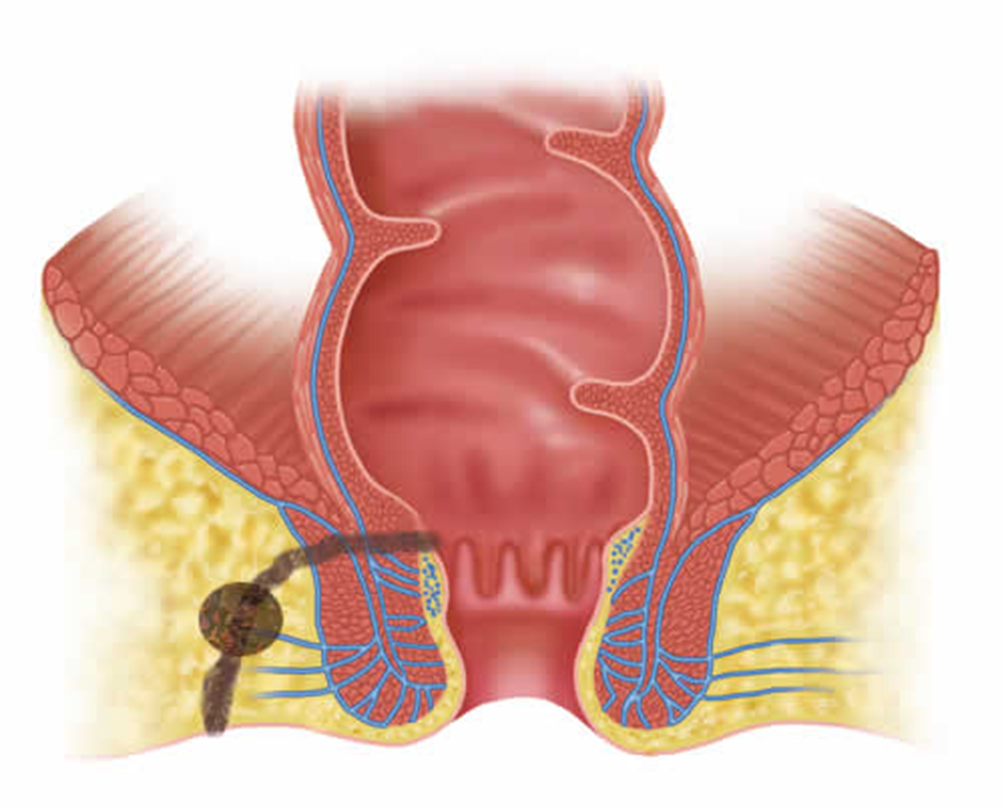
Figure 1
