Metastatic Breast Cancer Presenting as Gastric Linitis Plastica
Metastatic Breast Cancer Presenting as Gastric Linitis Plastica
Joseph AMARA[1,4]*, Santa EL HELOU[1,4], Clarisse KATTAN[2,4], Elio MIKHAEL[1,4], Rhea KHAZEN[3,4], César YAGHI[1,4], Joseph KATTAN[2,4]
(1) Hepatology and Gastroenterology unit at Hôtel-Dieu de France, Lebanon
(2) Hematology and Oncology unit at Hôtel-Dieu de France, Lebanon
(3) Pathology unit at Hôtel-Dieu de France, Lebanon
(4) University of Saint Joseph, Beirut, Lebanon
*Correspondence to: Joseph AMARA, resident at Hôtel-Dieu de France hospital in the Gastroenterology unit, affiliated to the University of Saint Joseph, Beirut, Lebanon.
Copyright
© 2024 Joseph AMARA. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 04 November 2024
Published: 18 November 2024
DOI: https://doi.org/10.5281/zenodo.14184955
Abstract
Linitis plastica is a diffuse thickening of the stomach resulting from the infiltration of the gastric submucosa and muscularis propria by neoplastic cells. The most common cause is gastric adenocarcinoma, followed by metastatic disease, most frequently breast cancer in its invasive lobular subtype.
We present the case of a 57yo woman, with a history of invasive ductal carcinoma (Hormone receptors positive, Her2 negative) treated 8 years ago and in remission, presented for epigastric pain and coffee ground emesis. She had two positive lymph nodes.
CT scan with IV contrast showed thickened gastric walls, with enlarged mesenteric lymph nodes and minimal ascites.
A gastroscopy showed micro-erosive and indurated mucosa with pyloric stenosis. Biopsy showed infiltration by independent signet-ring cells consistent with adenocarcinoma of mammary origin. Hormone and Her-2-neu receptors were negative.
The patient was diagnosed with metastatic triple negative breast cancer with gastric involvement in the form of linitis plastica. She was treated with Adriamycin 90mg and Carboplatin 450mg every 3 weeks.
This case shows linitis plastica as a metastatic recurrence of ductal carcinoma. The originality relies on the rarity of late breast cancer relapse in the form of linitis plastica, and its rare association with the invasive ductal subtype.
Keywords: Breast cancer, gastric cancer, endoscopy, linitis plastic.
Metastatic Breast Cancer Presenting as Gastric Linitis Plastica
Introduction
Linitis plastica represents a diffuse thickening and rigidity of the stomach that results from an inflammation of the gastric walls and subsequent fibrosis[1]. The pathophysiology behind this phenomenon is a diffuse infiltration of the gastric submucosa and muscularis propria by neoplastic cells[2].
The most common cause of linitis plastica is gastric adenocarcinoma. The second most common cause is a metastatic disease. Metastasis to the stomach occurs in only 0.2-0.7% of disseminated disease, with most frequently breast cancer as the origin (27.9% of the cases), followed by lung cancer (23.8%), esophageal carcinoma (19.1%), renal cell carcinoma (7.6%), and malignant melanoma (7.0%)[3]. Interestingly, the histologic subtype that is most associated with linitis plastica in the setting of metastatic breast cancer is the invasive lobular subtype[2].
We hereby present the case of a 57 years old woman who presented with linitis plastica as a metastatic recurrence of a previously localized invasive ductal carcinoma treated 8 years earlier. The originality of this case report not only relies on the rarity of late breast cancer relapse in the form of linitis plastica, but also it’s rare association with the invasive ductal subtype.
Case Presentation
A 57-year-old woman, known to have hypertension, diabetes and hypothyroidism, presented to the emergency department for severe epigastric pain, intractable coffee ground emesis and anorexia that has worsened in the last few days. Her past surgical history includes cholecystectomy, appendectomy, and hysterectomy for benign metrorrhagia.
She also had a left breast invasive ductal carcinoma that was treated eight years ago with mastectomy and lymph node dissection, followed by radiotherapy and adjuvant chemotherapy. Immunohistochemical staining of the breast cancer showed an Estrogen Receptor positive, Progesterone Receptor positive, and Her2 negative breast cancer with two positive lymph nodes. Further details concerning the chemotherapy received back then were not available.
At presentation, patient was stable, conscious, cooperative, and oriented upon admission. Physical exam was normal except for epigastric abdominal tenderness upon palpation. Laboratory tests were normal except for mild electrolytic disturbances suggestive of malnutrition and dehydration.
CT scan was performed with IV contrast injection and showed thickened gastric walls with innumerable enlarged mesenteric lymph nodes and minimal amount of fluid in the Douglas pouch. An upper gastrointestinal endoscopy was done and showed normal esophageal mucosa, congestive nodular fundic and antral mucosa (leopard skin appearance or carmin red lesions) with large thickened gastric folds that fail to distend on insufflation and impassable pyloric stenosis. Deep biopsies were taken in order to obtain sufficient histology materials.
Figure 1: Gastric mucosa showing heavy infiltration of the lamina propria by adenocarcinoma (A, H&E x100). High-power view reveals an independent signet-ring cell proliferation (B, H&E x400). Immunohistochemistry studies show positive staining with GATA3 (C, x200) and negative staining with CDX2 (D, x200)
Results
The first lecture of gastric biopsies concluded for linitis plastica of gastric adenocarcinoma. With her history of luminal breast cancer, a second anatomopathological look of the biopsy slides was performed in order to rule out relapse of breast cancer. The final results came back as follow: infiltration of independent signet ring cells with an immuhistochemical profile compatible with a breast cancer origin. Upon relapse, the hormone receptors as well as HER2 were negative. PDL1 was also negative with CPS score of less than 10 (Figure).
The patient was thus diagnosed with metastatic triple negative breast cancer with gastric involvement in the form of linitis plastica. Subsequently, she was treated with Adriamycin 90 mg and Carboplatin 450 mg every 3 weeks.
Discussion
Breast cancer is the most common cancer in females and the most common cause of cancer related death in women[4]. The usual sites of metastasis of breast cancer are the lungs, bones, liver and brain[5].
The gastrointestinal tract is an extremely rare site of metastasis with the stomach being involved in 0.2-0.7% of cases and the colon in only 0.1%. The invasive lobular carcinoma histologic subtype is incriminated in the majority of cases of metastasis to the stomach, whereas the invasive ductal carcinoma subtype is only responsible for a minority of the cases[6]. According to Taal et Al, among 56 patients who had breast cancer metastasized to the stomach, only 10 patients had invasive ductal carcinoma histology, as compared to 36 with invasive lobular carcinoma and 5 undifferentiated subtypes[7].
Moreover, there are three forms of metastasis to the stomach: gastric linitis, discrete nodules or external compression. Lobular breast cancer is associated commonly with linitis plastica whereas the more common ductal subtype is associated with a nodular form of gastric involvement[8].
Our patient had an invasive ductal carcinoma subtype that was associated to the gastric linitis.
Linitis plastica is defined as a rigidity and thickening of the gastric walls giving a ‘leather bottle’ appearance. It leads to symptoms such as dyspepsia, dysphagia, early satiety, post prandial vomiting and weight loss[9,10]. Diagnosis is done through imaging which shows diffuse gastric thickening and confirmed with a biopsy by endoscopy that shows diffuse proliferation of fibrous tissue in the submucosa. Involvement of the deeper layers of the gastric walls makes the diagnosis challenging as multiple deep biopsies are required to obtain proper tissue specimens[11].
Estrogen receptor (ER) and progesterone receptor (PR) are positive in about 80% of breast cancer. Even though these receptors can also be positive in gastric cancer in some cases (32% for ER and 12% for PR), their presence along with other markers is specific for a breast cancer origin[12]. In our case, the patient had negative estrogen, progesterone and Her2 markers on gastric biopsies.
The other markers that are specific for breast cancer origin include gross cystic disease fluid protein (GCDFP-15), mammaglobin, and GATA protein type 3. Breast cancer is also positive for CK7 and CK18 and negative for CK20(13). In our case, the patient immunohistochemical staining of gastric biopsies showed negativity for PDL-1 and CDX2, and the only positivity was for GATA3.
Treatment of gastric linitis largely depends on the underlying cause. Gastric linitis due to gastric adenocarcinoma should be treated surgically when possible. However, breast cancer metastasizing to the stomach should be treated systemically with either chemotherapy or hormonal therapy depending on the hormonal receptor status[3]. Our patient, who presented with triple negative breast cancer with gastric metastasis, was treated with Adriamycin and Carboplatin every 3 weeks, while immunotherapy associations were restricted to PDL-1 overexpression in both immune infiltrating cells and tumor cells, which was not the case for this patient.
Conclusion
This case shows linitis plastica as a metastatic recurrence of ductal carcinoma. The originality relies on the rarity of late breast cancer relapse in the form of linitis plastica, and its rare association with the invasive ductal subtype.
Conflicts of interest: The authors declare no competing interests.
References
1. Kanne JP, Mankoff DA, Baird GS, Minoshima S, Livingston RB. Gastric Linitis Plastica from Metastatic Breast Carcinoma: FDG and FES PET Appearances. Am J Roentgenol. 2007 Jun;188(6):W503–5.
2. Mantiero M, Faggioni G, Menichetti A, Fassan M, Guarneri V, Conte P. Gastric Linitis Plastica and Peritoneal Carcinomatosis as First Manifestations of Occult Breast Carcinoma: A Case Report and Literature Review. Case Rep Oncol Med. 2018 Jul 8;2018:4714708.
3. Geada L, Kantor M, Mohan K, Weingrad D, Nasiff LS, Geada L, et al. An Uncommon Presentation of a Common Disease: A Review of Gastric Metastasis From Breast Carcinoma. Cureus [Internet]. 2020 Dec 5 [cited 2023 Jul 12];12(12).
4. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and Mortality and Epidemiology of Breast Cancer in the World. Asian Pac J Cancer Prev. 2016;17(sup3):43–6.
5. Park M, Kim D, Ko S, Kim A, Mo K, Yoon H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int J Mol Sci. 2022 Jun 18;23(12):6806.
6. Kimchy AV, Umoren MD, Rosenberg JJ, Ilagan C, Nithagon P, Shafa S, et al. Breast Cancer Metastasis to the Gastrointestinal Tract With Unusual Endoscopic and Histologic Presentations. ACG Case Rep J. 2022 Dec;9(12):e00938.
7. Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89(11):2214–21.
8. Jones GE, Strauss DC, Forshaw MJ, Deere H, Mahedeva U, Mason RC. Breast cancer metastasis to the stomach may mimic primary gastric cancer: report of two cases and review of literature. World J Surg Oncol. 2007 Jul 9;5(1):75.
9. Jafferbhoy S, Shiwani H, Rustum Q. Managing Gastric Linitis Plastica. Sultan Qaboos Univ Med J. 2013 Aug;13(3):451–3.
10. El-Nakeep S, Kasi A. Linitis Plastica. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Jul 12]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK563294/
11. PharmD CAK MD. Cancer Therapy Advisor. 2017 [cited 2023 Jul 12]. Gastric Linitis Plastica Diagnosis Uses Endoscopic and Radiologic Studies Approach.
12. Matsui M, Kojima O, Kawakami S, Uehara Y, Takahashi T. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992 Sep 1;22(5):421–5.
13. Kaufmann O, Deidesheimer T, Muehlenberg M, Deicke P, Dietel M. Immunohistochemical differentiation of metastatic breast carcinomas from metastatic adenocarcinomas of other common primary sites. Histopathology. 1996 Sep;29(3):233–40.
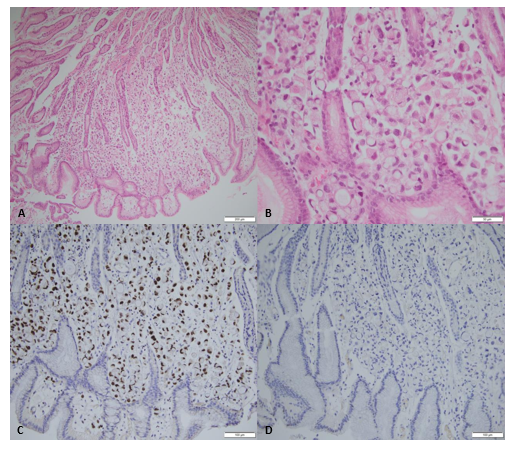
Figure 1
