Case Report: Pulmonary Embolism Induced by Mechanical Compression from Uterine Leiomyoma: A Rare Clinical Manifestation
Case Report: Pulmonary Embolism Induced by Mechanical Compression from Uterine Leiomyoma: A Rare Clinical Manifestation
Shahela Nasir *
*Correspondence to: Shahela Nasir MD (FCPS, MRCPI), Manchester University NHS Foundation Trust (MFT) UK.
Copyright
© 2024 Shahela Nasir. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 04 November 2024
Published: 19 November 2024
DOI: https://doi.org/10.5281/zenodo.14185568
Abstract
A 28-year-old female, six days post-cesarean section, presented with severe wound pain, fever, and night sweats. Clinical examination revealed necrotic tissue and signs of sepsis. Prompt surgical debridement and antibiotic therapy led to significant improvement and eventual discharge.
Case Report: Pulmonary Embolism Induced by Mechanical Compression from Uterine Leiomyoma: A Rare Clinical Manifestation
Introduction
Pulmonary Embolism (PE) is a sudden blockage in one of the pulmonary arteries in the lungs, commonly caused by blood clots that travel to the lungs from the legs or other parts of the body (deep vein thrombosis, or DVT).It is a life-threatening medical emergency that requires quick intervention and treatment. With symptoms that resemble many other medical conditions, including heart attack and pneumonia, it can be difficult to diagnose.1
Uterine fibroids, also called leiomyomas, are diagnosed in over two-third of females of reproductive age; about a quarter warrant treatment. Venous thromboembolism (VTE) is a challenging complication in patients with fibroids due to the increased risk of bleeding with anticoagulation, especially in the setting of associated menorrhagia. The incidence of deep vein thrombosis (DVT) is increased with higher uterine weight which may be related to the extrinsic venous compression of the iliac veins or the inferior vena cava.2
Case Report
I report a 47 yrs old lady P1 non smoker who presented to the emergency department. The initial presentation was with thigh erythema and elevated D-dimer required DVT exclusion, but the subsequent Doppler revealed a superficial thrombus rather than a deep venous clot. Started on Fondaparinux 2.5 mg subcutaneously daily for 6 weeks.
Her menstrual cycle was regular lasting 2 -3 days, unsure if heavy but often to use both tampon and pad. She felt bloated for sometime but no pressure, urinary and bowel symptoms. On examination normal observations, Chest clear, Normal I and II heart sounds with an additional systolic murmur. BMI The abdomen is distended with a large, palpable mass approximately 40 cm in diameter, hard, and non-tender.Mild swelling and tenderness to right thigh, no erythema. The incidental anaemia around 74 g/L with ferritin 2, was discovered on investigations, although asymptomatic. Low MCV and hematocrit indicate chronic anaemia. For further assessment of the mass CT thorax abdomen pelvis with contrast performed which revealed a heterogeneous intra-uterine collection, likely due to a large fibroid or possible hematometra. A further differential would be red degeneration of a large fibroid. CA125 raised to 103, though CT did not show malignancy. Fondaparinux has been stopped pending further assessment of possible
intra-uterine bleeding or until an MRI. A 1000mg ferrinject was administered for iron deficiency anaemia and 2nd dose prescribed.
Referred to Gynaecology for ultrasound to further assess the mass. US pelvis revealed there is a large heterogeneous mass measuring in excess of 207 x 153 x 133mm in the midline pelvis and extending superior to the umbilicus. Right ovarian hemorrhagic cyst noted. Planned for urgent outpatient MRI abdomen and pelvis with urgent outpatient gynae clinic appointment.
The MRI confirmed a large, degenerating posterior fibroid measuring approximately 156 x 129 x 196 mm, which is exerting mass effect on surrounding structures, including the right renal pelvis. There were no overly suspicious features for malignancy and no lymphadenopathy.
Given the patient's report of SOB( shortness of breath),in the context of recent superficial vein thrombophlebitis and a large abdominal mass warrants further investigation. The patient referred to A&E and advised to re commence fondaparinux. Same day the patient was seen in the emergency department.
CTPA revealed a filling defect in the right lower lobe pulmonary artery extending into multiple segmental branches. No signs of right heart strain were noted, which is favourable for prognosis.
Right sided PE confirmed. With a PESI (Pulmonary Embolism Severity Index) score of 47, the patient falls into a low-risk category for mortality from PE, suggesting that outpatient management could be appropriate with close monitoring.Prescribed apixaban 10 mg BD followed by 5 mg BD for minimum of 3 months. Outpatient follow up booked in the PE clinic and discussed increased risk of bleeding with apixaban.
Reviewed in the benign clinic, planned for total abdominal hysterectomy with bilateral salpingo oophorectomy. Menorrhagia worsened since the start of apixaban.On pre op assessment advised to stop apixaban 48 hours before surgery. Discussion done with a radiologist for the need of IVC (inferior vena cava) filter prior to surgery. Follow up in the PE clinic booked and plan to continue apixaban.
TAH & BSO performed uneventfully with operative findings of 32 weeks size large fibroid. Haematology planned prophylactic dalteparin for 48h post op, followed by treatment dose dalteparin for 7 days post op, then to restart apixaban. Uneventful recovery and patient discharged in stable condition.
Figure 1: CT thorax abdomen pelvis with contrast
Figure 2: Transvaginal Scan
Figure 3 : MRI
Figure 4: Intraoperative
Figure 5: CTPA revealed a filling defect in the right lower lobe pulmonary artery extending into multiple segmental branches
Discussion
Pulmonary thromboembolism is not a disease in and of itself. Rather, it is a complication of underlying venous thrombosis. Under normal conditions, microthrombi (tiny aggregates of red cells, platelets, and fibrin) are formed and lysed continually within the venous circulatory system. The classic presentation of PE is the abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia.1
Physical signs of pulmonary embolism include the following:
Tachypnea (respiratory rate >20/min): 96% Rales: 58% Accentuated second heart sound: 53% Tachycardia (heart rate >100/min): 44% Fever (temperature >37.8°C [100.04°F]): 43% Diaphoresis: 36% S 3 or S 4 gallop: 34% Clinical signs and symptoms suggesting thrombophlebitis:32% Lower extremity edema: 24% Cardiac murmur: 23% Cyanosis: 19%1.
Most PEs originate as lower extremity DVTs. Hence, PE risk factors are the same as DVT. Virchow's triad of hypercoagulability, venous stasis, and endothelial injury provides an understanding of these risk factors.
Pulmonary embolism (PE) is a major cause of mortality, morbidity, and hospitalisation. In 2021, PE was the underlying cause of death in 2638 people in England and Wales.
The mortality rate is lower in those who are hemodynamically stable and higher in those who present cardiorespiratory arrest. Severe cases of PE can lead to collapse and/or sudden death. About 5-10% of people present as hemodynamically unstable, and the risk of early death is greater than 15%.3
Of these cases, 34% died suddenly or within a few hours of the acute event before therapy could be initiated or take effect, 59% were deaths resulting from PE that remained undiagnosed during life, and 7% of the people who died early were correctly diagnosed with PE before death.4
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare and potentially fatal complication of PE, with a reported cumulative incidence of 0.1 -- 9.1% within the first 2 years after a symptomatic PE event.5
In people with CTEPH, fibrous tissue replaces residual emboli over months or years, leading to chronic obstruction of the pulmonary arterial vasculature, progressive increases in pulmonary arterial pressure, and, if untreated, right heart failure.
Risk factors include large thrombus mass, thrombophilic disorders, history of splenectomy, history of cancer, myeloproliferative disorders, inflammatory bowel disease, and previous episodes of VTE.6
For patients presenting with PE/VTE secondary to uterine fibroids, management also can be challenging, and the main goal for treatment is to deal with the VTE first and then remove the fibroid, as the ultimate goal is to address the PE before complications occur7.
A case reported by Alkhawam et al. (2024) discusses a woman with extensive bilateral PE linked to compression from a large fibroid, highlighting that uterine fibroids can indirectly lead to PE due to venous obstruction and reduced blood flow in the pelvic region.8
Fibroids increase venous stasis through direct compression. Large fibroids can exert a mass effect on the pelvic venous system and IVC, thus causing stasis in the pelvis and lower extremities. Venous stasis can also be caused by immobility or decreased activity during periods of heavy bleeding or debilitating pain and in the perioperative period for myomectomies and hysterectomies.9
Uterine leiomyoma is the most common benign pelvic tumours in women over age 30. Some of its well-known acute complications are thromboembolism, torsion of subserosal pedunculated leiomyoma, urinary retention, and haemorrhage. It is usually asymptomatic; however, as it increases in size, symptoms may present due to compression of the surrounding anatomic structures, resulting in significant increase in the incidence of thromboembolism. 7,10
If indeed uterine fibroids increase the risk of acute VTE, and namely acute PE, then there is a potential that uterine fibroids consequently increase the risk of developing chronic thromboembolic disease (CTED)/chronic thromboembolic pulmonary hypertension (CTEPH). Up to 4% of patients following an acute PE may develop CTEPH, with progressive increases in pulmonary vascular resistance (PVR) and pulmonary hypertension (PH) leading to right ventricular (RV) dysfunction, clinical right heart failure, and death.11
A case series of seven women who were diagnosed with CTED or CTEPH, and in the course of their evaluation, were found to have a history of large uterine fibroids compressing the pelvic venous system or IVC. Given that vascular compression is a known cause of thrombosis, my case findings suggest that large uterine fibroids leading to pelvic venous compression may represent a risk factor for VTE and eventual development of CTEPH.12 Moreover these five patients who are demonstrating approximately 71% of the cases underwent hysterectomy, with no subsequent VTE events after surgery. Additional suggestion was that fibroid compression was considered a significant factor in their original thromboembolic conditions.
Conclusion
PE linked to compression from a large fibroid, highlighting that uterine fibroids can indirectly lead to PE due to venous obstruction and reduced blood flow in the pelvic region.The patient was successfully treated with thrombolytic and anticoagulation therapy associated with total hysterectomy.
Despite treating the PE, it is essential to address the underlying cause of this pathology, so further investigations were warranted in our case, and an ultrasound of the pelvis was done to show intramural fibroid tumour. MRI of the abdomen was done for suspicion of compression effect, which truly revealed a presence of the fibroid compressing on iliac veins. surgical removal via hysterectomy which can be the definitive treatment to prevent the recurrence of VTE as was done in my case.
Such cases reinforce the need for clinicians to maintain a high index of suspicion for VTE in women with large fibroids,particularly those presenting with leg pain, dyspnea, or signs of venous insufficiency. The association between fibroids and VTE is considered a rare association and need for further research into preventive strategies and early detection methods.
The research is essential to further explore the relationship between uterine fibroids and pulmonary embolism, develop preventive strategies and management protocols.
Ethical Approval:
This case study has been published after a written informed consent from the patient, all related information and images have been kept strictly confidential and the publication is kept anonymous.
References
1- Ouellette, D.R., 2024. Pulmonary Embolism (PE). Medscape. Updated 10 July 2024. Edited by Z. Mosenifar. Available at: https://www.medscape.com/viewarticle/ 300901[Accessed 2 November 2024].
2- Latif, H., Baez Sosa, V., Farid, S., Fernandez, S., Hazen, N., Morozov, V., & Fitzpatrick, K.W. (2020) ‘Venous Thromboembolism in Women with Uterine Fibroids’, Blood, 136(Supplement 1),
pp. 28–29. Available at: https://doi.org/10.1182/blood-2020-136678
3- Konstantinides, S.V., Meyer, G., Becattini, C., et al. (2020) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European Heart Journal 41(4), 543-603.
4- Stein, P.D., Matta, F. and Firth, J.D. (2020) Deep venous thrombosis and pulmonary embolism. In: Firth, J., Conlon, C. and Cox, T. (Eds.) Oxford Textbook of Medicine. 6th edn. Oxford University Press.
5- ONS (2022) Deaths in 2019, 2021, and 2022 due to blood clots. Office for National Statistics. https://www.ons.gov.uk
6- NICE (2023) Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. National Institute for Health and Care Excellence. https://www.nice.org.uk
7-Gupta S, Manyonda IT. Acute complications of fibroids. Best Pract Res Clin Obstet Gynaecol 2009;23:609-17.
8- Alkhawam, M., Matthews, B., Chen, W., Sabouni, M.A., and McElwee, S.K. (2024) 'Extensive bilateral pulmonary embolism secondary to uterine fibroid induced iliac vein compression: case report', Preprints, [online] Available at: https://doi.org/10.20944/preprints202409.2238.v1 (Posted 27 September 2024).
9- Brown, D., Ghalib, N., Wu, J., Shahzadi, A., Simpson, T., Sharma, A., Bejugam, V.R., Soodhan, K., & Thachil, R. (2024) ‘Uterine Fibroid-Associated Massive Pulmonary Embolism: A Report of Two Cases’, Cureus, 15 June. Available at: https://doi.org/10.7759/cureus.62436
10- Shiota, M., Kotani, Y., Umemoto, M., et al. (2011) ‘Deep-vein thrombosis is associated with large uterine fibroids’, Tohoku Journal of Experimental Medicine, 224, pp. 87–89. doi: 10.1620/tjem.224.87.
11- Ende-Verhaar, Y.M., Cannegieter, S.C., Vonk Noordegraaf, A., et al., 2017. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. European Respiratory Journal, 49(2), p.1601792.
12- Lacharite-Roberge, A.S., Raza, F., Bashir, R., Dass, C.A., Moser, G.W., Auger, W.R., Toyoda, Y., Forfia, P.R. and Vaidya, A., 2018. Case series of seven women with uterine fibroids associated with venous thromboembolism and chronic thromboembolic disease. Pulmonary Circulation, 8(4), p.2045894018803873. Available at: https://doi.org/10.1177/2045894018803873
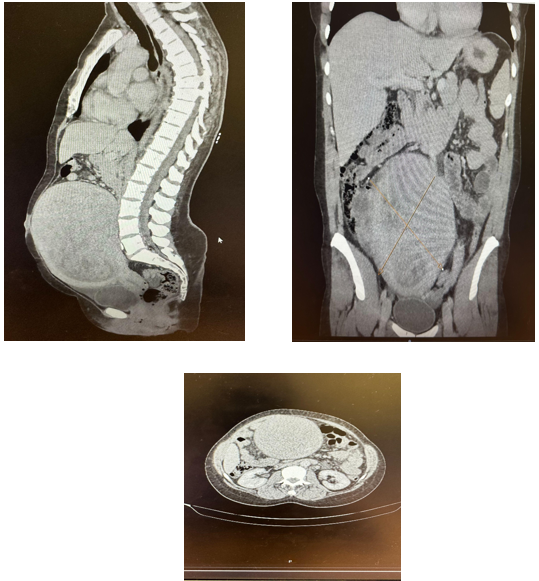
Figure 1
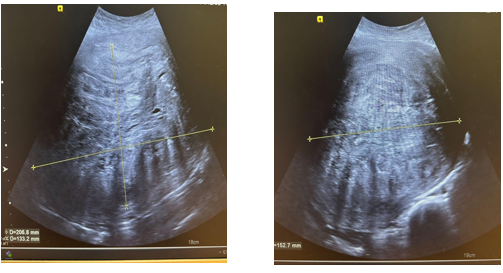
Figure 2
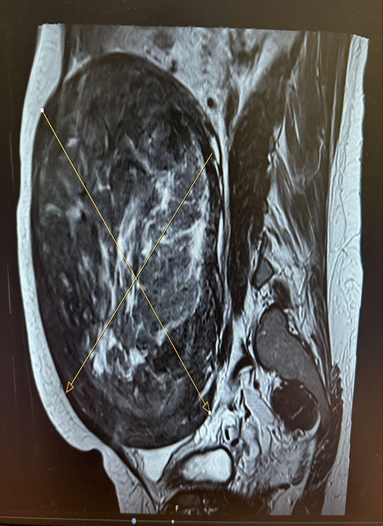
Figure 3
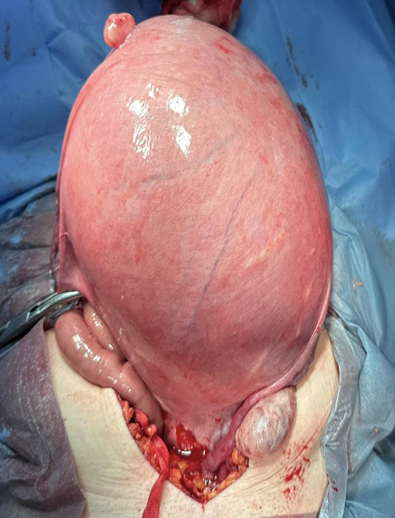
Figure 4
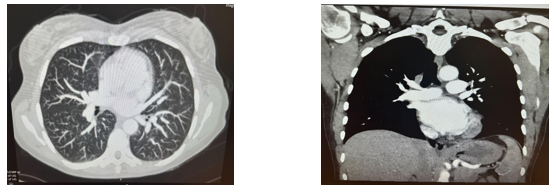
Figure 5
