Neonate with a Large Abdominal Cyst: Case Report and Review
Neonate with a Large Abdominal Cyst: Case Report and Review
Paras Khandhar*
*Correspondence to: Paras Khandhar, Department of Pediatrics, Division of Pediatric Critical Care Medicine, Corewell Health Children's Hospital, Royal Oak, MI.
Copyright
© 2024: Paras Khandhar. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 23 Nov 2024
Published: 01 Dec 2024
Abstract
Gastrointestinal duplication is a rare abnormality of the embryological development of the gastrointestinal tract, with an estimated incidence of 1/10,000 in live births and 1/4,500 in neonates and fetuses. It can appear in any portion of the intestinal tract, with the ileum being the most common location. A 5-day-old patient with a prenatal diagnosis of megacolon or mesenteric cyst was diagnosed during antenatal follow-up. A cystic mass attached to the terminal ileum was found approximately 5 cm from the ileocecal valve, which was resected and classified as Type 1B intestinal duplication according to the Long classification. Diagnosis is suggested by abdominal X-rays, ultrasonography, and computerized axial tomography scan with contrast studies, but the definitive diagnosis is made by histopathological examination of the surgical specimen.
Key Words: Child; Neonate; Intra-abdominal cyst; Enteric duplication.
Neonate with a Large Abdominal Cyst: Case Report and Review
Introduction
Fetal intra-abdominal cystic lesions are common and can be detected through prenatal ultrasound or abdominal complaints. In complex cases, CT and/or MRI can be helpful. Diagnosis should include choledochal, hepatic, ovarian, enteric (duplication), omental, mesenteric (duplication), renal, urachal or splenic cysts, hydronephrosis, urinoma formation, enlarged fetal urinary bladder, and lymphangiomas. Other differentials include a cystic appearing double bubble due to duodenal atresia, meconium pseudocyst, and hydrometrocolpos.
A cystic mass in a girl should first suggest an ovarian cyst, regardless of its location. In both sexes, digestive duplications, urachal and bile duct cysts are common if unicystic, and cystic lymphangioma if multicystic. Non-irradiating fetal MRI enhances diagnostic accuracy and can aid in characterizing the lesion in relation to surrounding anatomic structures. Fetal MRI has increased diagnostic accuracy for fetuses with abdominal cystic lesions from 51% to 73.4%, correcting the diagnosis or providing more precise information in 26.5% of cases.
In this case, a large antenatal intra-abdominal cystic lesion led to the final diagnosis of a gastrointestinal (enteric) duplication (GED). No laboratory tests or diagnostic procedures are generally necessary to diagnose mesenteric or omental cysts. Ultrasonography is the most useful imaging test, revealing fluid-filled cystic structures with thin internal septa and internal echoes from debris, hemorrhage, or infection.
Figure 1: CT-Scan Coronal and Axial planes of the abdominal cavity
Figure 2. Laparotomy findings
Figure 3. Gross macroscopic specimen
Figure 4. Histological analysis of the wall of the cyst
Case Report
A 2815g 5-day-old male patient was born at 37.5 weeks gestation with an APGAR score of 9-10-10. An ultrasound scan identified an intra-abdominal lesion, suggesting a megacolon diagnosis. After birth, further investigations revealed a large extrahepatic unilocular fluid-filled structure measuring 80x30 mm, indicating a mesenteric cyst, cystic lymphangioma, or composite hepatic hamartoma. A cystic mass attached to the terminal ileum was noted at laparotomy, and the cyst and intestine were closely related. The ileon-cyst complex was resected, and end-to-end interrupted primary anastomosis using vicryl 4/0 was performed. The gross pathological specimen showed a 75x10 mm intestinal fragment with ileal mucosa of normal appearance joined to a 80x35cm cystic structure containing mucus without luminal communication. The cyst contained mucus covered by glandular epithelial tissue and shared musculature with the intestinal portion, covered by gastric tissue. The cystic structure shared musculature with the intestinal portion, covered by gastric tissue within normal histological limits. According to the Long classification, the specimen was classified as Type 1B intestinal duplication. The patient's post-operative period was uneventful; at six months, he is thriving well without any complications.
Discussion and Conclusion
GED is a rare congenital anomaly that develops along the intestinal tube, with an estimated incidence of 1/10,000 in live births and 1/4,500 in neonates and fetuses. These anomalies are rare and occur with a frequency of 0.2% in children. They can appear in any portion of the intestinal tract from the neck to the rectum, with the most common site being the ileum. The incidence, according to location, is 20% in the esophagus, 4% thoracoabdominal, 7% gastric, 5% duodenal, 44% jejunoileal, 15% colonic, and 5% rectal. Of the total, 75% are cystic and 25% tubular. There can be synchronous (multiple) duplications up to 20%, although most report 5-10%.
In 1884, Reginald Fitz introduced the term "intestinal duplication" to describe and explain congenital cystic anomalies of the gastrointestinal tract, which he thought were remnants of the omphalomesenteric duct. In 1937, Ladd introduced the term digestive tract duplication (TD) to group congenital anomalies with three characteristics: 1- the presence of a well-developed wall of smooth muscle; 2- Epithelial lining representing some portions of the primary or heterotopic gastrointestinal tract, and 3- Most are attached to some portion of the digestive tract with or without communication to the intestinal lumen.
No single embryological theory satisfactorily explains the origin of all duplications. Various causes are assumed to be involved, such as failure of normal regression of the embryogenic diverticulum, traction of the endoderm and overlying structures during early embryogenesis, recanalization errors of epithelial connections within the small intestine, and adherence of the endodermal lining wall of the developing intestine to create a double lumen.
Intestinal duplications are gastrointestinal anomalies that can cause peptic ulcers in unlikely sites such as the ileum and posterior mediastinum. The wall of the duplicated segment is usually complete, with only rarely communicating with the intestinal lumen. Sometimes, the duplication forms an intrinsic part of the intestinal wall with fusion between the muscularis propria of the duplication and that of the normal intestine. The cystic lumen usually does not communicate with the intestinal lumen, and non-communicating cysts mimic the intestinal wall with enteric mucosa, submucosa, muscularis propria, and a myenteric plexus. Intramural duplications do not have a muscular layer but share the muscular layer with the adjacent intestine.
The clinical manifestations of intestinal duplications are nonspecific and depend on the type of duplication, its location, the presence of heterotopic gastric mucosa, and the complications it may cause. Cystic thoraco-enteric duplications are usually extramural and found in the posterior mediastinum, causing respiratory symptoms in infancy and communicating through the diaphragm with the intra-abdominal gastrointestinal tract. Abdominal duplications can present with abdominal pain, palpable mass, intestinal obstruction, melena, and even intussusception. Gastrointestinal bleeding occurs in 34% of cases due to mucosa ulceration or ischemia secondary to extrinsic compression of the normal intestine due to an adjacent cystic duplication. Intestinal obstruction can be due to intussusception, intestinal volvulus, or extrinsic compression from a cystic duplication.
Diagnosis of intestinal duplications is made using chest X-rays, ultrasonography (USG), CT computerized axial tomography, contrasted studies, and diagnostic laparoscopic techniques. In the prenatal period, antenatal ultrasound scans and magnetic resonance imaging can be used for diagnosis. Contrast radiological studies can also be used for differential diagnosis of mesenteric cysts, especially the cystic type. Histological examination is the definitive diagnosis.
The treatment of alimentary tract duplications is surgical, aiming for total resection due to the high risk of recurrence associated with partial excision or puncture. The operation should be elective and performed laparoscopically to relieve symptoms and prevent further complications. The treatment of choice is resection of the intestinal duplication with end-to-end anastomosis. Complications may be related to the size and location of the duplication, its communication with the gastrointestinal tract or vertebral canal, the presence of heterotopic gastric mucosa, and the involvement of mesenteric vessels. Future challenges include laparoscopically assisted procedures and externalizing the segment to be anastomosed.
References
1. Khong PL, Cheung SCW, Leong LLY, Ooi CGC, Ultrasonography of Intra-abdominal Cystic Lesions in the Newborn, Clinical Radiology, Volume 58, Issue 6,2003,Pages 449-454,ISSN 0009-9260, https://doi.org/10.1016/S0009-9260(03)00125-9.
2. Radswiki T, El-Feky M. Fetal intra-abdominal cysts (differential). Reference article, Radiopaedia.org. (accessed on 22 Aug 2022) https://doi.org/10.53347/rID-14733
3. Cass D L. Fetal abdominal tumors and cyst.Transl Pediatr 2021;10(5):1530-1541. http://dx.doi.org/10.21037/tp-20-440
4. Hugele F, Dumont C, Boulot P, et al. Does prenatal MRI enhance fetal diagnosis of intra-abdominal cysts? Prenat Diagn 2015;35:669-74
5. Saxena, K A, Mesenteric and Omental Cysts in Children: Practice Essentials, Pathophysiology, Etiology. Medscape 2022 Available at: https://emedicine.medscape.com/article/938463-overview [Accessed 12 Sep 2022]
6. Stringer MD. Duplication of the alimentary Tract. In: Puri P. Pediatric Surgery General Principles and Newborn Surgery. 1st edition. Berlin, Heidelberg: Springer; 2020. p.935-954.
7. Cárdenas MA, Vázquez F, Betancourth-Alvarenga J, Centeno M, Murcia FJ, Paredes R.M. Intestinal duplication, our experience. Cir Pediatr. 2016; 29: 54-57 Disponible en Disponible en: https://www.secipe.org/coldata/upload/revista/2016_29-2_54-57.pdf
8. Letelier M A, Barría MC, Beltrán MA, Moreno C. Intestinal duplication: Diagnosis and treatment of an unusual condition. Rev Chil Cir . 2009 ; 61( 2 ): 171-175. Disponible en: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0718-40262009000200011&lng=es. http://dx.doi.org/10.4067/S0718-40262009000200011.
9. Espino M. Chanis Águila R. Coronado A K. Modesto Dutari J. Intestinal duplication as an unusual cause of lower intestinal bleeding. Pediátr Panamá 2017; 46 (3): 50-53. Disponible en: http://docs.bvsalud.org/biblioref/2018/02/877525/2017-46-3-50-53.pdf
10. Russel KW, Holcomb III GW. Alimentary tract duplications. In:Holcomb III GW, Murphy PJ, Peter SDS. Holcomb and Ashcraft’s Pediatric Surgery. Seventh Edition. USA:Elsevier; 2020 p.629-640.
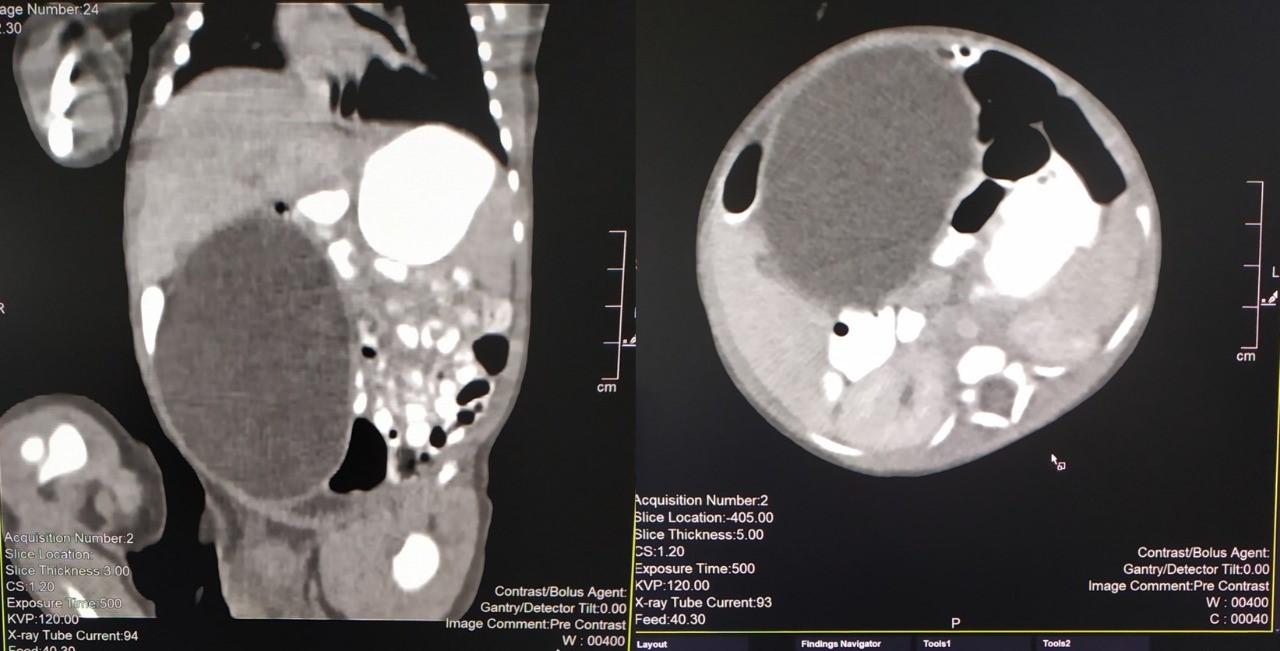
Figure 1
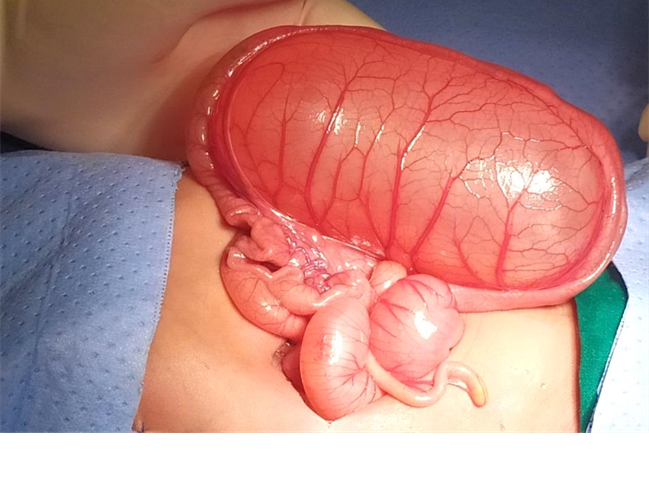
Figure 2
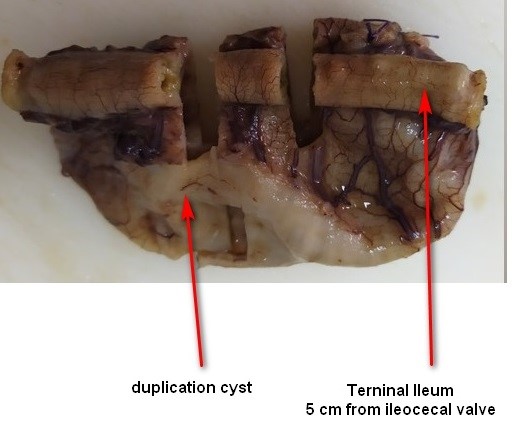
Figure 3
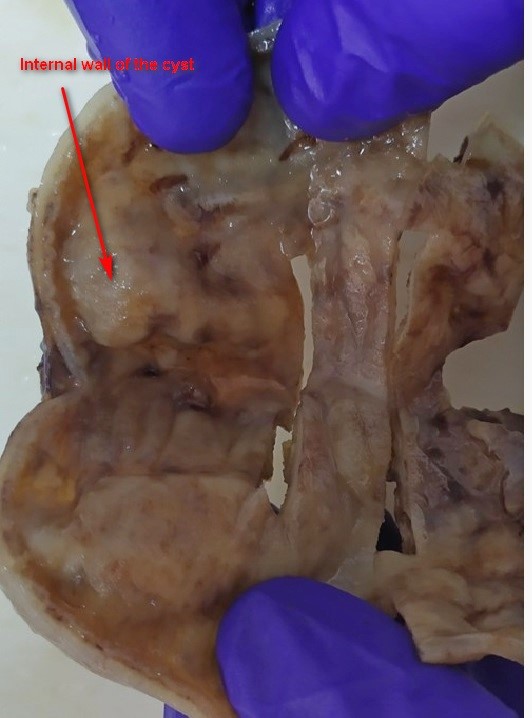
Figure 4
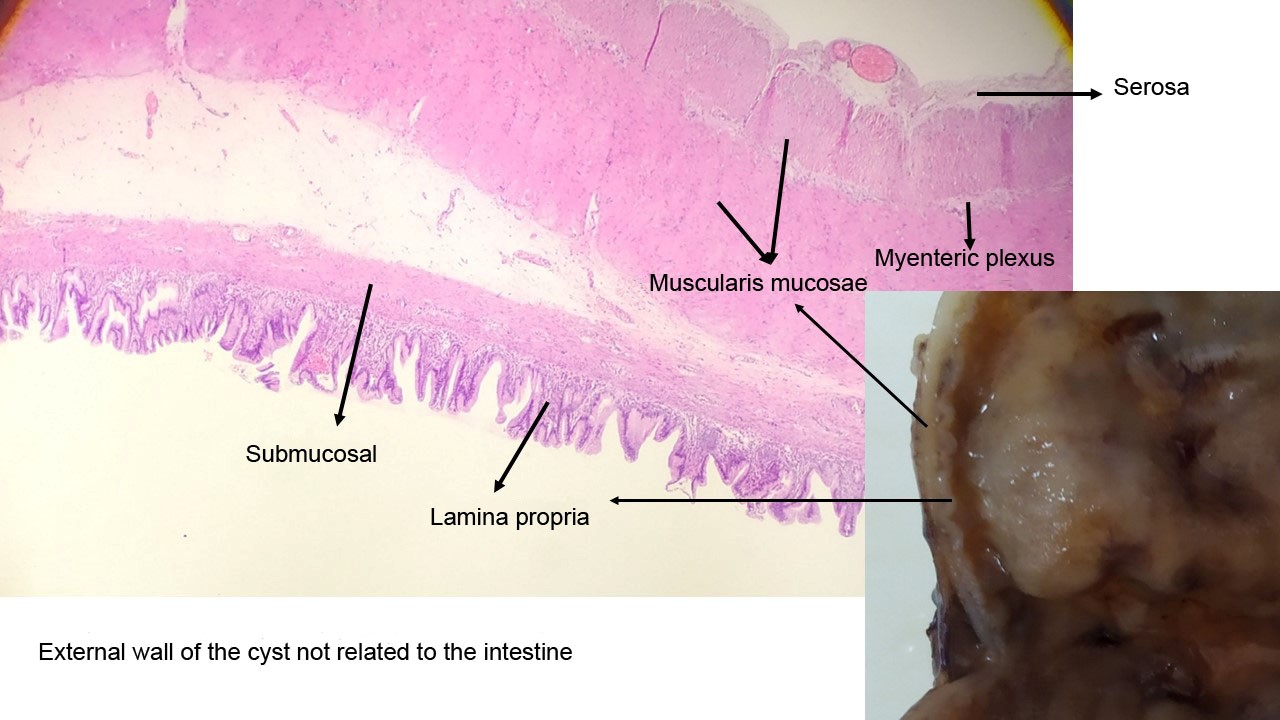
Figure 5
