An Investigation on the Prevention of Acute Pyelonephritis and Renal Impairment in Children with Vesico-Ureteric Reflux by Antibiotic Prophylaxis
An Investigation on the Prevention of Acute Pyelonephritis and Renal Impairment in Children with Vesico-Ureteric Reflux by Antibiotic Prophylaxis
Dr Monalisa Pradhan* , Dr Srinivasrao G Shinde1, Dr Hemant Yadav2
1. Dr Srinivasrao G Shinde Assistant Professor, Department of Paediatrics, ESIC Medical College & Hospital, Kalaburagi.
2. Dr Hemant Yadav Assistant Professor, Department of Paediatrics, Atal Bihari Vajpayee Government Medical College, Vidisha, M.P.
*Correspondence to: Dr Monalisa Pradhan, Senior Resident, Department of Neonatology, AIIMS, Bilaspur, H.P, India.
Copyright
© 2024: Dr Monalisa Pradhan. This is an open access article distributed under the Creative Commons AttributionLicense, which permits unrestricted use, distribution, and reproduction in any medium, provided the originalwork is properly cited.
Received: 04 Dec 2024
Published: 20 Dec 2024
Abstract
Urinary tract infections (UTIs) are common in young children, with fever often signaling renal involvement. Vesicoureteral reflux (VUR) predisposes children to acute pyelonephritis and potential renal scarring, which may lead to chronic renal complications. The efficacy of long-term antibiotic prophylaxis in children with VUR remains controversial, with limited and inconsistent evidence from prior studies. This prospective interventional study aimed to assess the effectiveness of sequential antibiotic prophylaxis in preventing recurrent UTIs and reducing renal scarring in children with VUR grades 2–4. Conducted at Department of Paediatrics, ESIC Medical College and Hospital, Kalaburagi, Karnataka, the study enrolled eligible children aged one day to five years after the first episode of acute pyelonephritis. Participants underwent three phases: baseline screening, sequential antibiotic prophylaxis with three different antibiotics, and 12 months of follow-up. Clinical and laboratory outcomes, including renal function, UTI recurrence, and renal scarring, were evaluated using a standardized statistical approach. The findings aim to contribute to evidence-based management of VUR in pediatric patients.
An Investigation on the Prevention of Acute Pyelonephritis and Renal Impairment in Children with Vesico-Ureteric Reflux by Antibiotic Prophylaxis
Introduction
Urinary tract infections (UTIs) are a frequent occurrence in young children, with fever often indicating renal parenchymal involvement. Renal damage during the acute phase of UTI may lead to scarring, a potential precursor to chronic renal failure. Vesicoureteral reflux (VUR) increases the risk of acute pyelonephritis by facilitating bacterial ascension to the upper urinary tract, where inflammatory responses may cause renal scarring.
The role of long-term antibiotic prophylaxis in preventing recurrent UTIs and new renal scars has been widely debated. Past studies evaluating its efficacy have been limited by poor design, heterogeneous populations, and insufficient evidence. While earlier treatment protocols advocated prophylaxis for all children with VUR, this practice was based more on expert opinion than robust scientific evidence. Uncertainty persists about the direct association between VUR, pyelonephritis recurrence, and renal scarring. Recent Cochrane reviews have highlighted the need for well-designed randomized studies to evaluate the efficacy of long-term antibiotic prophylaxis in preventing UTIs and subsequent renal damage in at-risk children.
This study aims to address these gaps by assessing the effectiveness of sequential antibiotic prophylaxis in reducing UTI recurrence and renal scarring in children with VUR grades 2–4, providing valuable insights into the management of this condition.
Methods
This prospective interventional study was conducted at Department of Paediatrics, ESIC Medical College and Hospital, Kalaburagi, from 2013–2014. The study included children aged one day to five years with VUR grades 2–4, diagnosed following an acute pyelonephritis episode or during antenatal screening.
Inclusion Criteria:
1. Unilateral or bilateral VUR grades 2, 3, or 4.
2. Diagnosed between one day and five years of age.
3. Gestational age > 35 weeks.
4. Glomerular filtration rate (GFR) > 15 ml/min/1.73 m².
5. Parental consent.
Exclusion Criteria:
1. VUR grade 1 or 5.
2. Gestational age < 35 weeks.
3. GFR < 15 ml/min/1.73 m².
4. Functional or structural abnormalities affecting urination (e.g., myelomeningocele).
5. Severe allergic reactions to study antibiotics.
6. Critical medical conditions preventing study participation.
Study Design:
The study was divided into three phases:
1. Baseline Screening: Measurements included height, weight, body mass index (BMI), blood pressure, serum creatinine, urine analysis and culture, renal ultrasound, and a renogram with DMSA.
2. Intervention: Participants received sequential antibiotic prophylaxis for 12 months. The antibiotics— Cefixime (2 mg/kg/day), Trimethoprim-Sulfamethoxazole (2.5 mg/kg/day based on trimethoprim), and Nitrofurantoin (1.5–2 mg/kg/day)—were administered in monthly cycles. Breakthrough UTIs were treatedwith appropriate therapeutic doses based on culture and sensitivity.
3. Follow-Up: Clinical and laboratory evaluations were conducted every three months, including blood pressure, urine analysis, serum creatinine, and renal ultrasound. After 12 months, assessments included a renogram, BMI, and voiding cystourethrography.
Statistical analysis was performed using SPSS v22. Descriptive and inferential tests, including normality tests, frequencies, and independent sample t-tests, were used. Results were evaluated with a significance threshold of 0.05 to determine statistical relationships.
Results
The study focuses on evaluating various clinical outcomes related to urinary tract infections (UTIs) in patients over a 12-month period. Key objectives include assessing the incidence and recurrence of UTIs, the development of renal scars, the persistence of vesicoureteral reflux, the onset of hypertension, proteinuria, and deterioration of renal function. The study also monitors body mass index (BMI) and identifies bacterial pathogens causing UTIs, along with their antibiotic sensitivity and resistance profiles. Primary outcomes involve determining UTI incidence and renal scarring, while secondary outcomes include other complications such as hypertension, proteinuria, and renal function decline. Evaluation methods include imaging studies, voiding cystourethrograms (VCUG), urine culture, serum creatinine measurements, and BMI calculations. This comprehensive approach aims to improve understanding of UTI complications and guide effective
Figure 1, 2
Discussion
Study Adherence and Population:
- Out of 60 eligible patients, only 30 (50%) adhered to the preventive antibiotic regimen, compared to 90% in the PRIVENT study and 76% in the RIVUR study.
Follow-Up Period:
- The study followed patients for 12 months, similar to PRIVENT but shorter than the RIVUR study (24 months) and other studies (up to 5 years).
Patient Demographics:
- Mean age was 30.73 months, older than PRIVENT (14 months) and RIVUR (12 months).
Baseline Urinary Sepsis:
- Initial urinary sepsis rate was 60%, compared to 71% in PRIVENT and 91% in RIVUR, with international studies reporting rates as low as 20%-28% in severe reflux cases.
Gender Distribution:
- Females constituted 53.3% of patients, aligning with most studies but lower than PRIVENT (64%) and RIVUR (92%).
Urinary Symptoms:
- Symptoms of lower urinary infections were present in 86.7% of cases, contrasting with 12%-16% in RIVUR, where upper infections predominated.
Vesicoureteral Reflux (VUR):
- 80% had known VUR at the start, similar to RIVUR (80.4%), but higher than PRIVENT (42%).
Family and Perinatal History:
- Family history of VUR was reported in 6.7% of cases, lower than other studies (27%-45%). Positive perinatal history was present in 13.3%, suggesting a potential risk factor for future research.
Antibiotic Usage:
- Unjustified antibiotic use was high (66.7% before admission), highlighting concerns over chaotic prescribing practices.
Growth and Renal Function:
- Children's BMI remained normal, indicating no adverse effects on growth. Preventive antibiotics reduced renal damage and urinary infections significantly.
Renal Scarring and Hydronephrosis:
- Renal scars were present in 70% of patients initially, much higher than RIVUR (3.6%) and PRIVENT (25%). Hydronephrosis affected 66.6% of patients, contrasting with RIVUR (5.3%).
Urine Cultures and Bacterial Resistance:
- Positive urine cultures dropped to 16.6% after 3 months, and no bacterial resistance was observed, unlike RIVUR, where resistance to specific antibiotics was 63%.
UTI Relapse and Antibiotic Breakthroughs:
- UTI relapse occurred in 20% of patients, higher than PRIVENT (13%) and RIVUR (10%).
Regression of VUR:
- Significant improvement in VUR grades, especially grade 4, occurred during follow-up, highlighting the potential role of antibiotics in VUR regression.
Impact on Renal Scars and Hydronephrosis:
- No effect on renal scar or hydronephrosis regression, consistent with RIVUR findings.
Overall, the study highlighted differences in demographics, adherence, and outcomes compared to PRIVENT and RIVUR. The findings suggest potential benefits of preventive antibiotics in managing VUR and reducing infections but emphasize the need for longer follow-up and larger sample sizes to draw robust conclusions.
Conclusion
The application of preventive antibiotics with a single evening dose for a period of one year in patients with vesicoureteral reflux of grades (2,3,4) led to the prevention of recurrent urinary infections, and also led to the decline of renal damage and improvement of renal function significantly, while it did not affect the occurrence or progression of renal scars.
References
1. Comprehensive pediatric nephrology 1st ed. Geary D F, Schaefer F. Vesicoureteral Reflux. Ranjiv M, Mattoo T K; 2008 (36): 549 –56.
2. WHITE B, MD. Diagnosis and Treatment of Urinary Tract Infections in Children. Am Fam Physician; 2011 Feb 15; 83(4): 409-15.
3. Mattoo T K, MD. Are prophylactic antibiotics indicated after a urinary tract infection? Curr Opin Pediatr. 2009 April; 21(2): 203–06.
4. Wheeler D, Vimalachandra D, Craig J C et al. Antibiotics and surgery for vesicoureteric reflux: a meta- analysis of randomized controlled trials. Arch Dis Child 2003 (88): 688–94.
5. Dillon M J, Goonasekera C D. Reflux nephropathy. J Am Soc Nephrol; 1998 (9): 2377-83.
6. Robinson J. Antibiotic prophylaxis in vesicoureteral reflux. Can Pharm J (Ott); Mar 2013; 146(2): 84–87.
7. Nagler E V, Williams G, Craig J C et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev 2011 (6): CD001532.
8. Allen U D, MacDonald N, Fuite L et al. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMA J 1999 (160): 1436-40.
9. Mori R, Fitzgerald A, Williams C et al. Antibiotic prophylaxis for children at risk of developing urinary tract infection: a systematic review. Acta Paediatr 2009 (98): 1781-6.
10. Mattoo T K. Evidence for and against urinary prophylaxis in vesicoureteral reflux. Pediatr Nephrol 2010
(25): 2379-82.
11. American Urological Association. Management and screening of primary vesicoureteral reflux in children: AUA guideline. Linthicum, MD. American Urological Association; 2010.
12. Gargollo P C, Diamond D A. Therapy Insight: what nephrologists need to know about primary vesicoureteral reflux. Nature Clinical Practice Nephrology; 2007 (3): 551-63.
13. Urinary tract infection in children: Diagnosis, treatment and long-term management. National Institute for Health and Care Excellence (NICE); Aug 2007. www.nice.org.uk/guidance/CG54/chapter/1-Guidance.
14. Judkins A, Pascoe E, Payne D. Management of urinary tract infection in a tertiary children’s hospital before and after publication of the NICE guidelines. Arch Dis Child; 2013 (98): 521-5.
15. Lytzen R, Thorup J, Cortes D. Experience with the NICE guidelines for imaging studies in children with first pyelonephritis. Eur J Pediatr Surg; 2011 (21): 283-6.
16. Hoberman A, Greenfield S P, Carpenter M A et al. Antimicrobial Prophylaxis for Children with Vesicoureteral Reflux. N Engl J Med; 2014; 370(25): 2367-76.
17. Keren R, Shaikh N, Hoberman A et al. Risk Factors or Recurrent Urinary Tract Infection and Renal Scarring. Pediatrics; 2015; 136(1): 13-21.
18. Cheng C H, Tsai M H, Lin T Y et al. Antibiotic Resistance Patterns of Community-Acquired Urinary Tract Infections in Children with Vesicoureteral Reflux Receiving Prophylactic Antibiotic Therapy. Pediatrics Dec 2008; 122 (6): 1212-7.
19. Garin E H, Olavarria F, Young L et al. Clinical Significance of Primary Vesicoureteral Reflux and Urinary Antibiotic Prophylaxis After Acute Pyelonephritis: A Multicenter, Randomized, Controlled Study. Pediatrics Mar 2006; 117 (3): 626-32.
20. Craig J C, Simpson J M, Williams G J. Randomized Intervention for Children with Vesicoureteral Reflux. N Engl J Med 2009; (361): 1748-59.
21. Hoberman A, Greenfield S P, Mattoo T K et al. Randomized Intervention for Children with Vesicoureteral Reflux. N Engl J Med 2014; (370): 2367-76.
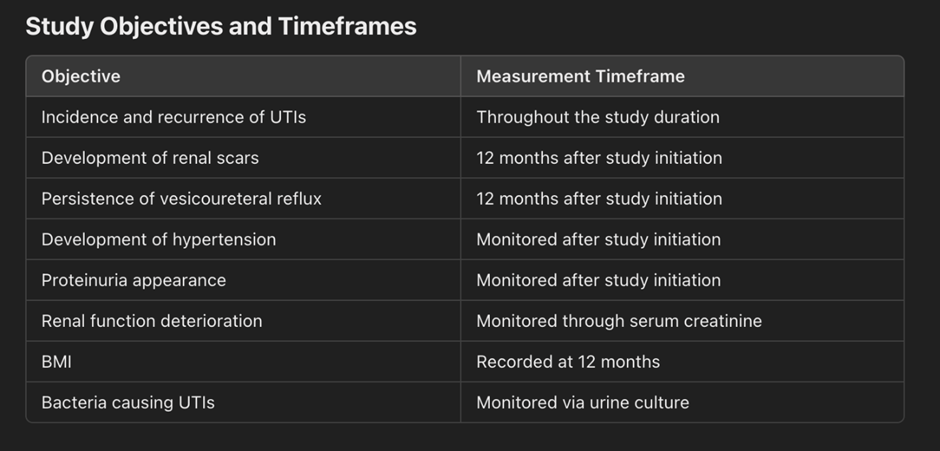
Figure 1
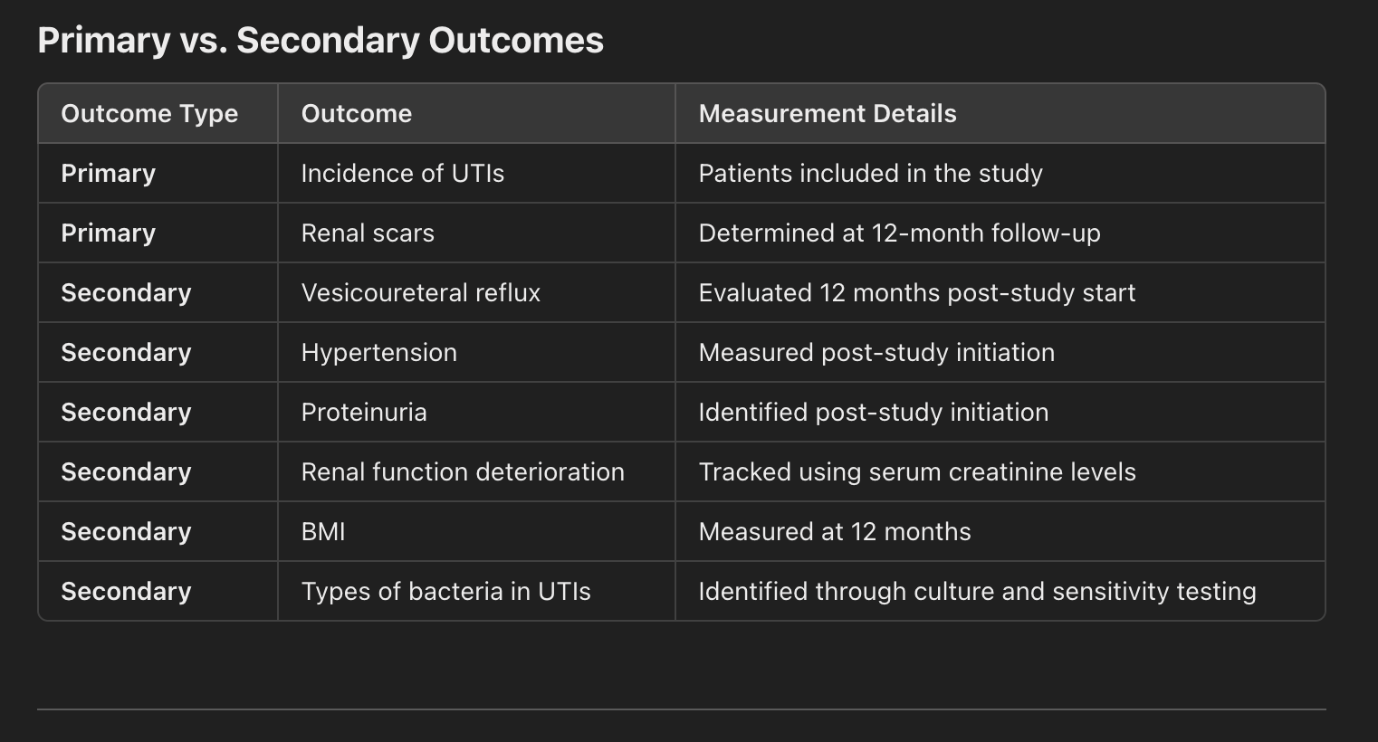
Figure 2
