Short Term Outcomes of the Patients Undergoing Allogenic Hematopoietic Stem Cell transplant for Acute Myeloid Leukemia; A Single Center Experience from developing World
Short Term Outcomes of the Patients Undergoing Allogenic Hematopoietic Stem Cell transplant for Acute Myeloid Leukemia; A Single Center Experience from developing World
Rubina Khan1, Yasir Abbass2, Mehreen Ali Khan3, Hira Tariq4, Muhammad Yousaf5*, Raheel Iftikhar6
1,2,3,4,5,6. Armed Forces Bone Marrow Transplant Centre, Rawalpindi, Pakistan.
*Correspondence to: Muhammad Yousaf, Armed Forces Bone Marrow Transplant Centre, Rawalpindi, Pakistan
Copyright.
© 2025 Muhammad Yousaf. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 17 December 2024
Published: 02 January 2025
DOI: https://doi.org/10.5281/zenodo.14859060
Abstract
Background: Acute myeloid leukemia (AML) accounts for approximately 1% of all cancers. Despite the advancements in chemotherapy and targeted treatments, hematopoietic stem cell transplantation (HSCT) remains the only potentially curative option for patients with for relapsed refractory AML. This study aims to evaluate the short-term outcomes of patients undergoing HSCT for AML.
Methodology: This cross-sectional study was conducted at the AFBMTC from January 2022 to December 2023. Participants were selected through a convenient, non-probability sampling method. Ethical approval was obtained from the institutional review board of AFBMTC. Written informed consent was obtained from all patients or their legal guardians for both the transplant procedure and the use of their data for research purposes. Data was extracted from hospital record. Data was analyzed using SPSS version 23. Qualitative variables were presented as frequencies and percentages. Quantitative variables were summarized as medians with interquartile ranges (IQR) or means with standard deviations (SD). Kaplan-Meier survival analysis was used to estimate OS and DFS.
Results: A total of 41 patients underwent HSCT during the defined study duration and all were included in the study. There were 26 (63.4%) males and 15 (36.6%) females. The mean age of the patients was 24.46 ± 11.6 years. Thirty-eight patients (92.7%) were diagnosed with de novo AML, 2 (4.9%) progressed from MDS, and 1 (2.4%) had therapy-related AML. Risk stratification was available for 36 patients in which 9 (22%) had favorable risk category, 25 (61%), in relapsed refractory disease, intermediate and 2 (4.9%) were high risk category. Among all patient who underwent HSCT, 34 (82.9%) patients had a matched sibling donor (MSD) and 7 (17.1%) had haplo matched donor available. CMV reactivation was demonstrated in 16 (39%) patients while among 23 (56.1%) patient no CMV reactivation was recorded by the last day of follow-up. All of the patients received Myelo-ablative conditioning chemotherapy. the OS was 63.4% with mean survival of 281.71 + 112 days DFS was 46.3 % with mean survival of 269 + 123 days. By using log rank test, Mucositis had significant association with OS (P=0.001) and DFS (P=0.003).
Conclusion: Our study highlights that allogeneic HSCT with myeloablative conditioning achieves substantial short-term yields favorable engraftment and survival outcomes in patients with AML patients, with a 1-year overall survival rate of 63.4% and disease-free survival of 46.3%. Mucositis was prevalent in 80.5% of patients, was associated with significantly lower survival rates, highlighting the critical impact of early complications on overall outcomes. Additionally, the high incidence of acute and chronic GVHD (36.6% and 22%, respectively) and CMV reactivation (39%) highlights the need for refined prophylactic measures to reduce these complications. Future studies with larger, diverse populations and comprehensive molecular profiling are needed to refine these findings and guide tailored post-transplant strategies.
Keywords: Acute Myeloid Leukemia, Allogenic HSCT, Short-Term outcomes.
Short Term Outcomes of the Patients Undergoing Allogenic Hematopoietic Stem Cell transplant for Acute Myeloid Leukemia; A Single Center Experience from developing World
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy characterized by accumulation of clonal, abnormally undifferentiated myeloid progenitors in the peripheral blood, bone marrow, or any other organ, leading to impaired hematopoiesis and bone marrow failure.[i] AML accounts for approximately 1% of all cancers.[ii] The most recent age-adjusted global incidence estimate of AML was 1.54 per 100,000 person-years.[iii] In South Asia the highest incidence rate of acute leukemia was in Pakistan 4.3 per 100,000 and Sri Lanka 4.1 per 100,000 and the lowest in Bangladesh 1.8 per 100,000.[iv] Despite the advancements in chemotherapy and targeted treatments, hematopoietic stem cell transplantation (HSCT) remains the only potentially curative option for patients with for intermediate and high-risk AML, particularly those in first complete remission (CR1) or with relapsed/refractory disease.[v]
HSCT offers a chance for long-term remission by providing the graft-versus-leukemia (GVL) effect, where donor immune cells recognize and eliminate residual leukemia cells. However, this effect is associated with significant risks, including graft-versus-host disease (GVHD), infections, and treatment-related mortality, all of which alter short-term outcomes of HSCT. Recent studies are focusing to reduce the conditioning intensity to improving transplant-related outcomes by tailoring conditioning intensity, better donor selection, and advances in supportive care. Despite all this the early post-transplant period remains critical, with high rates of mortality and morbidity. Short-term outcomes, serve as an important indicator of overall success of HSCT.
Literature regarding treatment outcomes of HSCT in AML in Pakistan is scarce. There is very limited data highlighting the outcomes in this population, which makes it difficult to improve patient survival. This study aims to evaluate the short-term outcomes of patients undergoing HSCT for AML, examining key factors such as engraftment rates, relapse incidence, non-relapse mortality, and overall survival post-transplant. By identifying clinical and biological variables associated with these outcomes, we try to contribute to the development of more effective strategies for managing high-risk AML patients undergoing HSCT.
Methodology
This cross-sectional study was conducted at the Armed Forces Bone Marrow Transplant Center (AFBMTC) from January 2022 to December 2023. All patients diagnosed with acute myeloid leukemia (AML), regardless of age, who underwent HSCT, were included. Participants were selected through a convenient, non-probability sampling method, meaning all eligible patients during the study period were enrolled. Ethical approval was obtained from the institutional review board of AFBMTC. Written informed consent was obtained from all patients or their legal guardians for both the transplant procedure and the use of their data for research purposes. The study was conducted in accordance with the Declaration of Helsinki and all relevant local guidelines
All patients were diagnosed with AML based on WHO criteria and were considered for HSCT after achieving complete remission through chemotherapy. Patients who had incomplete data in the registry or lost to follow up before completing one year post HSCT, were excluded from the analysis. Data were collected retrospectively from the AFBMTC patient registry, which includes detailed transplant-related information. The main variables studied included:
1. Mucositis: Graded according to the World Health Organization (WHO) oral toxicity scale.
2. Neutrophil Engraftment: Defined as three consecutive days with an absolute neutrophil count (ANC) ≥ 0.5 x 10?/L.
3. Platelet Engraftment: Defined as the first day with a platelet count ≥ 20 x 10?/L without transfusion support for at least seven days.
4. Cytomegalovirus (CMV) Reactivation: Monitored using weekly CMV PCR testing post-transplantation.
5. Graft-versus-Host Disease (GVHD): Graded using the Magic criteria for acute and chronic GVHD.
6. Disease-Free Survival (DFS): Time from HSCT to relapse or death from AML
7. Overall Survival (OS): Time from HSCT to death from any cause or completion of 1 year post HSCT
All patients underwent Myeloablative regimens conditioning regimens according to the transplant protocol at AFBMTC. HSCT was performed by bone marrow harvest, as main source of graft, along with peripheral blood stem cells (PBSCs) if dose is not sufficient. Donors were matched related siblings in all cases. CD34+ cell count in the graft was quantified, and TNC count was recorded.
Post-transplant follow-up included routine monitoring for early complications such as mucositis, infections, and engraftment delays. CMV reactivation was tested weekly using quantitative PCR, and patients with reactivation were treated with antiviral therapy. Acute and chronic GVHD was monitored and categorized based on clinical and laboratory parameters.
All data was analyzed using SPSS version 23. Qualitative variables, such as the occurrence of mucositis, GVHD, and CMV reactivation, were presented as frequencies and percentages. Quantitative variables, including CD34+ cell dose, TNC count, time to neutrophil engraftment, and time to platelet engraftment, were summarized as medians with interquartile ranges (IQR) or means with standard deviations (SD), depending on the distribution of the data. Kaplan-Meier survival analysis was used to estimate OS and DFS. Survival curves were generated and compared using the log-rank test. Statistical significance was set at p < 0.05. Cox proportional hazards models were used for multivariate analysis to identify factors independently associated with survival outcomes.
Results
A total of 41 patients underwent HSCT during the defined study duration and all were included in the study. There were 26 (63.4%) males and 15 (36.6%) females. The mean age of the patients was 24.46 ± 11.6 years. Thirty-eight patients (92.7%) were diagnosed with de novo AML while 2 (4.9%) progressed from MDS, and 1 (2.4%) had therapy-related AML. As per ELN risk stratification was available for 36 patients in which 9 (22%) had showed favorable risk category, 25 (61%) intermediate and 2 (4.9%) were high risk category. The demographics of the patients included in the study are given in table 1.
Table 1; Demographics of the patients with Acute Myeloid leukemia undergoing HSCT
|
|
Frequency |
Percentage |
|
Gender of the Patients |
||
|
Male |
26 |
63.4 |
|
Female |
15 |
36.6 |
|
Age of the Patients |
||
|
<12 Years |
5 |
12.2 |
|
13-30 Years |
25 |
61.0 |
|
>30 Years |
11 |
26.8 |
|
Primary Diagnosis of the Patients |
||
|
AML-Mo |
2 |
4.9 |
|
AML-M1 |
6 |
14.6 |
|
AML-M2 |
18 |
43.9 |
|
AML-M3 |
3 |
7.3 |
|
AML-M4 |
4 |
9.8 |
|
AML-M5 |
3 |
7.3 |
|
AML-M6 |
2 |
4.9 |
|
AML-MRC |
2 |
4.9 |
|
t-AML |
1 |
2.4 |
|
Risk Stratification of the Patients as per ELN classification |
||
|
Favorable |
9 |
22.0 |
|
Intermediate |
25 |
61.0 |
|
Adverse |
2 |
4.9 |
|
Record Not available |
5 |
12.2 |
|
NPMI Status of the Patients |
||
|
Positive |
5 |
12.2 |
|
Negative |
36 |
87.8 |
|
FLT3 Status of the Patients |
||
|
Positive |
1 |
2.4 |
|
Negative |
40 |
97.6 |
|
BCR-ABL1 Status of the Patients |
||
|
Positive |
0 |
0 |
|
Negative |
5 |
12.2 |
|
Not available |
36 |
87.8 |
|
Genetic Mutations Detected |
||
|
TP53 |
1 |
2.4 |
|
CBFB/MYH!! |
1 |
2.4 |
|
DEK-NUP214 |
1 |
2.4 |
|
D835 Mutation |
1 |
2.4 |
|
Negative/None |
37 |
90.2 |
|
Karyotyping of the patients |
||
|
Normal |
29 |
70.7 |
|
Not available |
2 |
4.9 |
|
Culture Failed |
3 |
7.3 |
|
Complex Karyotype |
1 |
2.4 |
|
t(8::21) |
1 |
2.4 |
|
Monosomy 8 |
1 |
2.4 |
|
t(11::17) |
1 |
2.4 |
|
t(6::9) |
1 |
2.4 |
|
Inversion 16 |
1 |
2.4 |
|
Hyperdiploid with Complex Karyotype |
1 |
2.4 |
Patient falling in either of the risk stratification category received induction chemotherapy at different institutes and remission assessment was done. Being intermediate or high risk disease at onset or poor response (Primary refractory disease) remained the base line to take the patient for HSCT. The main indication for HSCT among the patient underwent transplant during study duration, was being in intermediate risk category i.e. 18 (43.9%) or relapsed disease 14 (34.1%). Remission assessment along with minimal residual disease (MRD) was done in a few patients only. Among all patient who underwent HSCT, 34 (82.9%) patients had a matched sibling donor (MSD) and 7 (17.1%) had haplo matched donor available. All of the patients received Myelo-ablative conditioning chemotherapy. The Stem cell source was bone marrow harvest in 25 patients (61%) while 10(24.4%) received peripheral blood stem cells and 6 patients (14.6%) had both BMH and PBSC as source of stem cell transplant. Para meters assessed regarding HSCT are shown in the table 2.
Table 2: Parameter assessed prior to Hematopoietic Stem Cell Transplant
|
Indication of HSCT |
||
|
Primary Refractory Disease |
6 |
14.6 |
|
Relapse Disease |
14 |
34.1 |
|
Intermediate Risk Disease |
18 |
43.9 |
|
High Risk Disease |
1 |
2.4 |
|
Not Available |
2 |
4.9 |
|
Disease remission status prior to HSCT |
||
|
MRD Positive, Morphologically Complete Remission |
1 |
2.4 |
|
MRD Negative, Morphologically Complete Remission |
1 |
2.4 |
|
MRD Not available, Morphologically Complete Remission |
39 |
95.1 |
|
Type of HSCT |
||
|
Matched Sibling Donor HSCT |
34 |
82.9 |
|
Haplo-identical HSCT |
7 |
17.1 |
|
Source of Stem Cell collection |
||
|
Bone Marrow Harvest (BMH) |
25 |
61.0 |
|
Peripheral Blood Stem Cell collection (PBSC) |
10 |
24.4 |
|
BMH + PBSC |
6 |
14.6 |
For GVHD prophylaxis all fully match patients receive cyclosporine with methotrexate and those who underwent haplo-identical transplant received post-transplant cyclophosphamide. Median CD34 dose was 3.73 x 106 cells/kg (IQR: 2.90 to 4.35106 cells/kg) and TNC was 4.94 x 108 cells/Kg (IQR: 3.50 to 5.40). The mean days for neutrophil and platelet engraftment were 10.8 + 5.3 days and 20.25 + 6.3 days respectively. Regarding early complications of Allo-HSCT, Mucositis was one of the commonest complication majority patients experienced. A total of 33 (80.5%) patient developed mucositis post HSCT out of which majority had grade I or II. From Day 14 post HSCT every patient was screened for CMV reactivation fortnightly. CMV reactivation was demonstrated in 16 (39%) patients while among 23 (56.1%) patient no CMV reactivation was recorded by the last day of follow-up. CMV viremia was treated effectively with Val-ganciclovir was started once CMV copies were greater than 2,000. 15 patients out of 41 developed Acute GVHD, out of which majority had limited Skin GVHD of Grade I. Four patient needed hospitalization for grade IV GVHD. Early complications post HSCT are shown below in graph 1 and 2.
Graph 1: Post Allogenic early complication (Fig 5)
Graph 2: Grading of Acute GVHD and Mucositis (Fig 6)
When the data was further analyzed 9 (22%) patients developed chronic GVHD out of which 5 (55.5%) had limited disease and 4 had extensive GVHD. Among those having extensive GVHD 3 received Donor Lymphocyte Infusion (DLI) for relapsing disease. 10 (24.4) patients relapsed post-transplant, those who relapsed 3 patients received DLI after re-induction chemotherapy, 2 patients were started on palliative management and 1 patient underwent 2nd HSCT from a haplo identical sibling. By using kaplan meier, out of 41 patients the overall survival rate was 63.4% with mean survival of 281.71 + 112 days (Figure 1), with maximum survival of 60% among intermediate risk category. Out of 41 patients the disease-free survival rate was 46.3 % with mean survival of 269 + 123 days. By using log rank test, Mucositis had significant association with OS (P=0.001) and DFS (P=0.003) shown in figure 3 and 4.
Figure 1: Overall Survival
Figure 2: Disease Free Survival
Figure 3: Overall Survival VS Mucositis
Figure 4: Disease Free Survival VS Mucositis
Discussion
Our study assessed the short-term outcomes among patients with a primary diagnosis of AML who underwent myeloablative conditioning with busulfan and fludarabine, followed by allogeneic HSCT. Our study reports 1-year OS and DFS of 63.4% and 46.3% respectively. The OS of our center is comparable to the 5-year OS reported in a study from another developing country i.e. Iran and comparable results were reported by Mitus et al. as well. [vi],[vii] Previous studies have reported mean survival times in AML patients without prior transplantation ranging from 14 to 17 months. The results showed that OS and DFS of patients after allo-HSCT were reduces as the duration to the transplant increases and few other studies reinstate these findings, such as Frazer et al. states an OS of 60%, 45.5%, and 37.5% at 1, 3, and 5 years post-transplant respectively.[viii]While our study reports mean survival time post-transplant is 281.71 + 112 days, which might declines if the long term survivals are measured.
The occurrence of acute and chronic GVHD remains a significant complication, with 36.6% of patients developing acute GVHD and 22% experiencing chronic GVHD. Additionally, we observed a high incidence of CMV reactivation (39%), highlighting the toxicity associated with the conditioning regimen and emphasizing the necessity for effective monitoring and timely intervention. Moreover, the strong association between mucositis and both OS and DFS suggests that minimizing mucositis may improve patient outcomes. In a multivariate analysis indicated that OS has significant relationship with presenting WBC count with relapse.[ix] Similarly a number of studies supports this finding suggesting that the high WBC count at presentation is an unfavorable prognostic factor for treatment outcome in AML patients.[x] In contrast to previous studies, our analysis revealed that WBC count had a non-significant impact on OS and DFS in AML patients. This might be because majority of the patients underwent transplant during the defined study duration, presented with pancytopenia rather than elevated counts. Our findings also indicate that mucositis significantly impacts OS and DFS in the post-transplant setting.
The mean age of patients at the time of transplantation serves as another prognostic factor associated with survival. [xi] Increasing age is often linked to altered disease dynamics and adverse cytogenetic profiles, leading older adults to be less able to tolerate intensive therapy and more likely to be managed supportively.[xii] In our cohort, the mean age at transplantation was 24.46 ± 11.6 years, No significant relationship between age at transplant time and survival was detected (P=0.80) in the study. It was observed although non-significant but the younger age group had better OS and DFS as compared to older strata. While most studies indicate that individuals over 60 years have poorer survival rates compared to younger populations, our institutional policy sets the upper age limit for transplantation at 45 years, resulting in a lack of data for patients older than 60.
AML relapse after allo-HSCT predicted poor survival. Which is still a major therapeutic challenge in dealing patients with AML. A study by the European Blood and Marrow Transplantation (EBMT) group showed cumulative incidence of relapse after allo-SCT 32% ± 1% among AML patients. The longer intervals from transplant to relapse, low bone marrow tumor burden at relapse, and the absence of aGVHD were identified as prognostic factors associated with survival improvement in these patients.[xiii] Similar to the other studies we observed poor survival following AML relapse after allo-HSCT.[xiv] The results indicate that a hazard of death score in patients who had relapsed was 5.66 times worse than patients who had not relapsed. In our study we had 24.4% replace with in first years of HSCT which might increase if longer follow ups were made. One possible reason of such high relapse rate could be not assessing patients for minimal residual disease prior to taking them to transplant.
Limitations
Our study has several limitations. As the study duration was limited the relatively small sample size limits its generalizability. Absence of the cytogenetic and molecular studies, in most of the cases, are the most important limitations of this study. Patients referred to our center from different part of the country and these tests were not available or covered financially at their hospitals. Furthermore, while the follow-up period was substantial, it may not capture all late-onset complications or long-term outcomes. The single-center design may introduce biases related to specific clinical practices and patient populations.
Conclusion
Our study highlights that allogeneic HSCT with myeloablative conditioning achieves substantial short-term yields favorable engraftment and survival outcomes in patients with AML patients, with a 1-year overall survival rate of 63.4% and disease-free survival of 46.3%. Despite the constraints of a single-center, resource-limited setting, these outcomes are consistent with similar studies in developing region and align with international standards. Mucositis was prevalent in 80.5% of patients, was associated with significantly lower survival rates (p=0.001 for OS, p=0.003 for DFS), highlighting the critical impact of early complications on overall outcomes. Notably, mucositis emerged as a significant predictor of survival in this study, suggesting that interventions to prevent this complication may enhance the survival outcomes. Additionally, the high incidence of acute and chronic GVHD (36.6% and 22%, respectively) and CMV reactivation (39%) highlights the need for refined prophylactic measures to reduce these complications. Our findings also suggest that relapse remains a critical challenge, with relapsed patients exhibiting a hazard of death 5.66 times higher than those in remission. This may be partly due to limited MRD assessment capabilities, which highlights an area for improvement in patient selection and post-transplant surveillance. Future studies with larger, diverse populations and comprehensive molecular profiling are needed to refine these findings and guide tailored post-transplant strategies.
References
[i] Meillon-Garcia LA, Demichelis-Gómez R. Access to therapy for acute myeloid leukemia in the developing world: Barriers and solutions. Curr Oncol Rep [Internet]. 2020;22(12). Available from: http://dx.doi.org/10.1007/s11912-020-00987-8
[ii] Cancer statistics center [Internet]. American Cancer Society. 2023 [cited 2024 Jun 15]
[iii] Kristo F, Merinopoulou E, Radhakrishnan A, Joseph C, Dalal MR. POSA179 global incidence and survival of acute myeloid leukemia with 20-29% blasts: A systematic literature review
[iv] Incidence M, Of Leukemia in South E. Incidence, Mortality, and Epidemiology of Leukemia in South Asia: An Ecological Study. An Ecological Study
[v] Nataraj KS, Prabhu S, Bhat S, Badiger S, Vasundhara PK, Annapandian VM, et al. Hematopoietic stem cell transplant outcomes in patients with acute myeloid leukemia from a tertiary care centre in south India. Biol Blood Marrow Transplant [Internet]. 2020;26(3):S123–4
[vi] Mehdizadeh M, Bolourian V, Zamani G, Tavakoli-Ardakanii M, Zamani S, Tabarraee M, Hajifathali A. Survival of Patients with Acute Myeloid Leukemia after Allogeneic Stem Cell Transplantation: An Experience in Developing Country. Int J Hematol Oncol Stem Cell Res. 2022 Jan 1;16(1):55-65. doi: 10.18502/ijhoscr.v16i1.8443. PMID: 35975120; PMCID: PMC9339122.
[vii] Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74
[viii] Baron F, Labopin M, Niederwieser D, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26(12):2462–8.
[ix] Padilha SL, Souza EJ, Matos MC, et al. Acute myeloid leukemia: survival analysis of patients at a university hospital of Parana. Rev Bras Hematol Hemoter. 2015;37(1):21–7.
[x] Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant . 2015;21(3):454–9
[xi] Risk Factors Influencing the Survival of Pediatric Acute Leukemia Using Competing Risk Model
[xii] Kiss TL, Sabry W, Lazarus HM, Lipton JH. Blood and marrow transplantation in elderly acute myeloid leukaemia patients - older certainly is not better.
[xiii] Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep. 2016;6:1–7.
[xiv] Hecker J, Miller I, Gotze KS, Verbeek M. Bridging Strategies to Allogeneic Transplant for Older AML Patients. Cancers (Basel) . 2018;10(7):232
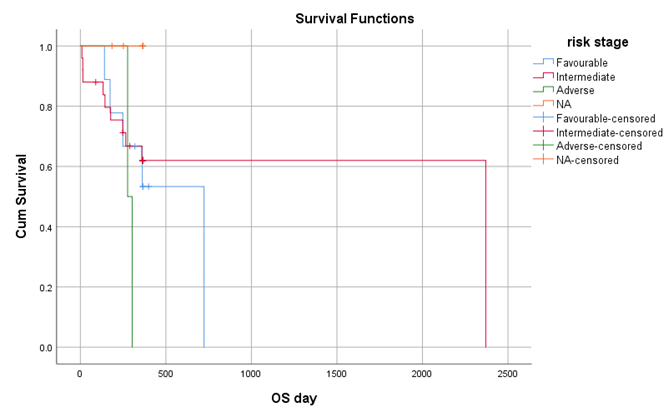
Figure 1
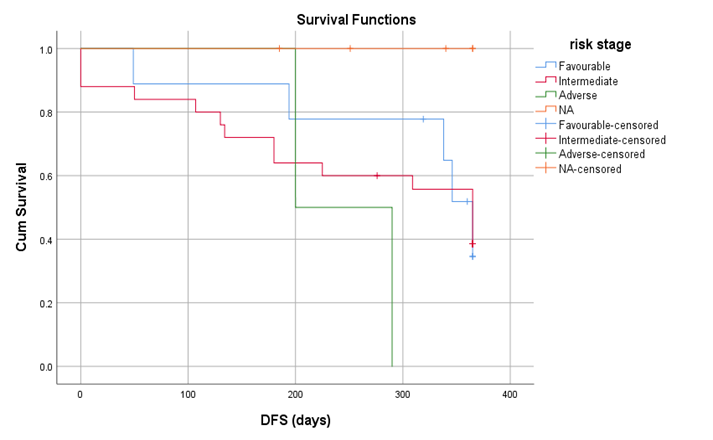
Figure 2
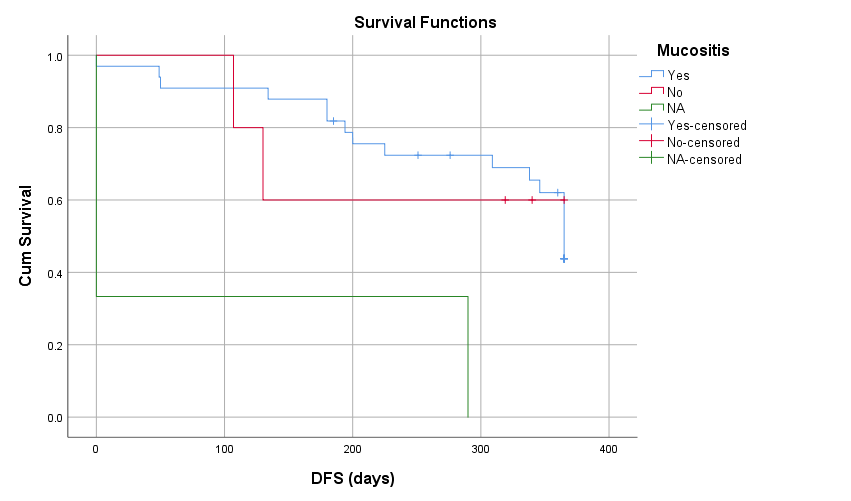
Figure 3
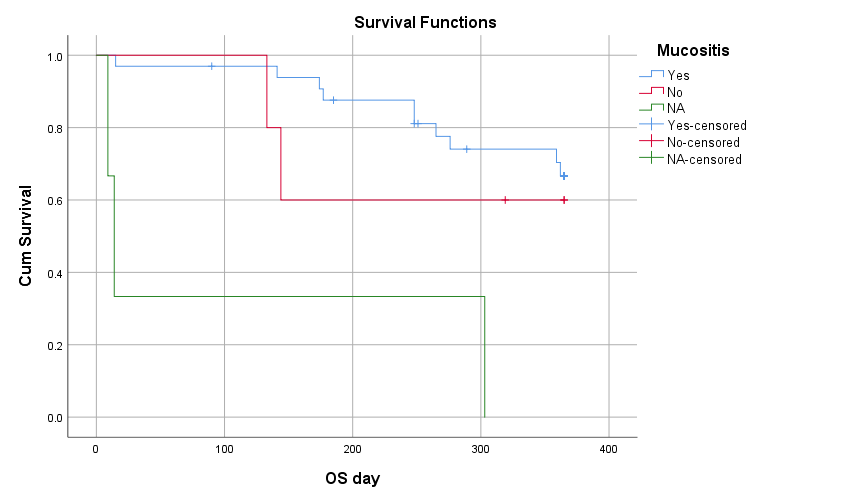
Figure 4
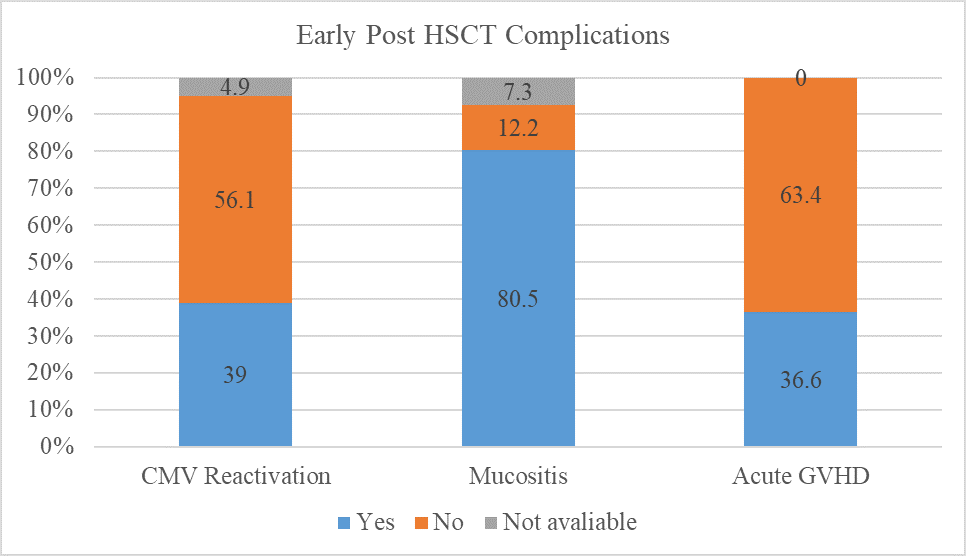
Figure 5
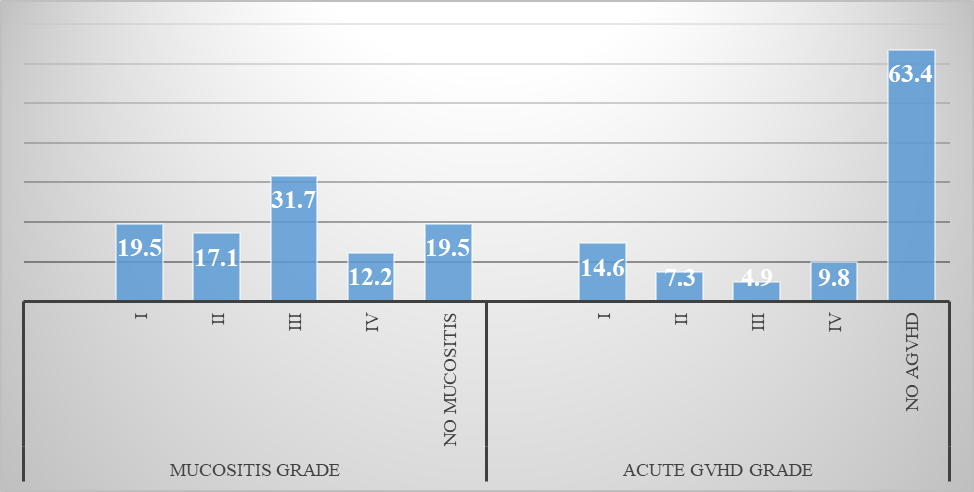
Figure 6
