Ewing’s Sarcoma of the Kidney: Case Series on an Overlooked Masquerade in Differential Diagnosis of Renal Cell Carcinoma
Ewing’s Sarcoma of the Kidney: Case Series on an Overlooked Masquerade in Differential Diagnosis of Renal Cell Carcinoma
Adeel Ahmed, Syed1*, Pervaiz, Arsalan2, Iqbal, Ather 3, Mubashir Nawaz, Muhammad 4, Shakeel, Saqib5, Usman Khan Mohammad6, Mir ,Khurram7
1,2,3,4,5,6,7. Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore Pakistan.
*Correspondence to: Adeel Ahmed, Syed, Shaukat Khanum Memorial Cancer Hospital and Research Centre, Lahore Pakistan.
Copyright
© 2024: Adeel Ahmed, Syed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 01 July 2024
Published: 20 July 2024
Abstract
Background: Primitive neuroectodermal tumor (PNET) of the kidney is an extremely rare entity and has got a very poor prognosis. Multimodal therapy, comprising of multi-agent chemotherapy with surgery and, or radiation therapy is the current therapeutic approach in management of renal PNET. Herein, we present a review of the clinical data of patients and follow up for the survival status of patients with primary renal PNET in our center.
Methods : We retrospectively analyzed the clinical data of patients with renal PNET diagnosed pathologically at SKMH & RC from January 1, 2009 to December 2023. All of the patients were followed up for survival status
Results: Twelve patients with renal PNET presented during the study period. The ratio of males to females was 10:2. The median age was 27.5 years (21–36 years) at the time of diagnosis. Only one patient was diagnosed with retroperitoneal lymph node metastasis. The main clinical manifestations of patients were flank pain (8/12), hematuria in (5/12) and fever (2/12). All but two patients developed metastasis on follow up .The Median metastasis free survival was 18.95 months .Seven patients died in our follow-up, with an average overall survival (OS) of 44.9 months.
Conclusions: As a rare renal tumor, renal PNET has a propensity to occur in young males. Most patients have distant metastasis when they are diagnosed, and the prognosis is very poor. Effective treatments like targeted anti-tumor agents are urgently needed.
Keywords: Renal primitive neuroectodermal tumor (renal PNET), renal cancer, case series, Ewing’s sarcoma
Ewing’s Sarcoma of the Kidney: Case Series on an Overlooked Masquerade in Differential Diagnosis of Renal Cell Carcinoma
Introduction
The Primitive neuroendocrine tumors (PNET) consists of a group of small round blue cell tumors which includes both osseous and extra-osseous lesions. Ewing sarcoma (EWS) and soft tissue primitive neuro-ectodermal tumors (PNET) are generally poorly differentiated tumors1 They are highly aggressive sarcomas leading to death and unfortunately commonly seen in young adults 2.
Retroperitoneal Ewing sarcomas are extremely rare, less than 5% of renal tumors3. The clinical manifestations and imaging findings of renal PNET are almost similar to those of other renal cell cancers due to which these tumors are easily mistaken for other more common tumors involving the kidney. Seemayer et al. reported the first case of PNET in 1975 4.
There is no consensus on the optimal management of renal PNET due to low prevalence and limited available data. The current management approach rests on multimodal therapy, including multi-agent chemotherapy with surgery and, or radiation therapy 5-7. The clinical and histopathological features of these tumors have been described widely in most published case series8-10, but only a few studies have described the management and patient outcomes 11. Available reports indicated that the majority of patients with renal PNET have an advanced disease at presentation with poor prognosis12,13.
The purpose of our study is to describe the clinical characteristics, treatment, and outcomes of patients with renal PNET at our institution.
Materials and Methods
We retrospectively reviewed the patients who presented with a renal mass and were diagnosed with histologically proven PNET between January 2009 till December 2023 at Shaukat Khanum cancer memorial hospital and research center (Lahore). After getting IRB approval medical records were reviewed retrospectively for patient’s demographics including age, gender, side and size of renal mass. Information regarding treatment, and outcome of patients were also collected.
The median free survival (MFS) and overall survival (OS) were calculated from the time of diagnosis. Local failure was defined as relapse within the operative bed and abdomen outside the operative bed. Tumor spread to other viscera was considered as distant metastasis. OS and MFS was estimated using Kaplan-Meier Curves. All statistical analyses was performed using SPSS version 2020.
Results
A total of 12 patients were diagnosed with histologically proven PNET during the study period.
The most common presenting symptoms were flank or abdominal pain (66.6%) and hematuria (41.6%). Two (16.6%) patients presented with systemic symptoms such as fever or weight loss.
The mean age of the patients was 27.5 years (range 21-36). Mean tumor size was 15.4 cm (range, 8–31 cm).Majority of the patients had tumor confined to the kidney. Tumor extension to the renal vein was present in two patients and only one patient had tumor extension to the inferior vena cava (IVC). One patient had a locally advanced disease at presentation. None of the patients had metastatic disease at diagnosis. (Table 1)
TABLE :1 Clinical Characteristics of Patients with Primary PNET of the Kidney
|
S.No |
Characteristic |
N (%) |
|
1. |
Age at diagnosis, years |
|
|
|
Mean |
27.5 (21-36) |
|
2. |
Gender |
|
|
|
Male |
10 (83.4) |
|
3. |
Tumor size, cm |
|
|
|
Mean |
15.45 (8-31) |
|
4. |
Tumor Extension |
|
|
|
Confined to kidney |
8 (66.66) |
|
|
Renal vein thrombus / IVC thrombus |
3 (25) |
|
|
Locally advanced |
1 (8.34) |
|
5. |
Side of tumor |
|
|
|
Right |
6 (50) |
|
6. |
Treatment |
|
|
|
Upfront surgery |
12(100) |
|
|
Adjuvant Chemotherapy |
12(100) |
|
|
Adjuvant Chemo-Radiotherapy |
3(25) |
All the patients had upfront surgery with adjuvant chemotherapy. The most common initial chemotherapy regimen used was vincristine, doxorubicin, and cyclophosphamide (VDC) in combination with ifosfamide and etoposide (IE). Three patients received post-operative radiation. (Table 2)
All but two patients developed metastasis on follow up. The most common sites of metastasis were pulmonary (n = 6, 50%), local bed recurrence (n = 5, 41.6%) and osseous metastasis (n=3, 25%). Two patients had brain metastasis. While metastasis to the liver was found in only one patient. (Table 2). The Median metastasis free survival was 18.95 months. (Figure1). Seven patients died in our follow-up, with an average overall survival (OS) of 44.9 months. (Figure 2)
TABLE: 02 Clinical characteristics, treatment and outcomes of patients with primary PNET of the kidney.
|
S.No |
Age in years |
Gender |
Side |
Size in cm |
Tumor Extension |
Nephrectomy |
Adjuvant Treatment |
Site of First Relapse, Time from Diagnosis |
Patient Outcome |
|
1. |
28 |
male |
Right |
14 |
Confined to kidney |
Upfront |
Chemo(VIDE, C,Topo) |
Renal Bed /1 month |
Death , 13 months |
|
2. |
35 |
Male |
Right |
10 |
Confined to kidney |
Upfront |
Chemo (VCDE/gem Dox) |
Brain ,Pulmonary/ 43 months |
Death , 110 months |
|
3. |
36 |
Male |
Left |
10 |
Confined to kidney |
Upfront |
Chemo (VIDE/VAC /VCE) |
Pulmonary ,Peritoneal , Renal bed/ 5 months |
Death , 88 months |
|
4. |
31 |
Male |
Left |
17 |
Confined to kidney |
Upfront |
Chemo (VCDE/VAC) |
None |
Alive , NED |
|
5. |
21 |
Male |
Right |
18 |
Confined to kidney |
Upfront |
Chemo(VIDE) |
Renal Bed/2 months |
Death 5 months |
|
6. |
27 |
Male |
Right |
18 |
Confined to kidney |
Upfront |
Chemo( VIDE/VCDE) |
Pulmonary, osseous ,renal bed/2 months |
Death , 13 months |
|
7. |
25 |
Male |
Left |
22 |
Confined to kidney |
Upfront |
Chemo(VIDE) |
Renal Bed /1 month |
Death 27 months |
|
8. |
29 |
Male |
Left |
8 |
Renal vein Invasion |
Upfront |
Chemo(VCD/IE/GEM/Doce) |
Pulmonary /15 months |
AWD |
|
9. |
21 |
male |
Left |
9.5 |
Confined to kidney |
Upfront |
Chemo (VDC/IE) |
None |
Alive, NED |
|
10. |
25 |
Female |
Left |
18 |
Renal vein Invasion |
Upfront |
Chemo(VDC/ IE) |
Hepatic , Osseous metastasis/ 1 month |
Death , 6 months |
|
11. |
28 |
Female |
Right |
10 |
Tumor in IVC |
Upfront |
Chemo (VDC/IE) |
Pulmonary /1 month |
Alive , NED |
|
12. |
24 |
Male |
Right |
31 |
Locally advanced |
Upfront |
Chemo (VDC) |
Brain, Pulmonary, Hepatic, Osseous/1 month |
AWD |
Abbreviations AWD-Alive with disease, C-Cyclophosphamide, D-Doxorubicin, E-Etoposide, I-Ifosfamide, IVC-Inferior vena cava, NED-No evidence of disease, , RT-Radiation therapy, RV-Renal vein, V-Vincristine, Gem –Gemcitabine , Doce –Docetaxel.
Figure 1 : kaplan-meier Showing Metastasis free survival
Figure 2: kaplan-meier Showing Overall survival
Discussion
Primitive neuroectodermal tumors (PNET) are rare sarcomas, especially those found primarily in the kidney. Renal PNET is commonly seen in young adults. The clinical manifestations and imaging findings of renal PNET are almost similar to those of other renal cell carcinomas. Renal PNET often present as a single, large mass on imaging14. Recently cases of primary renal PNET have been reported more frequently, mainly due to improved molecular studies.
In this study, we reviewed our experience with 12 patients with PNET of the kidney who were seen at our institution over a period of 14years. In this series, we observed a male to female preponderance (83.3%) as seen previously. The median age at diagnosis was 27.5 years. PNET in generally diagnosed at a younger age of usually less than 20 years, our age distribution is somewhat different15,16. Similar to previous reports the presenting symptoms of primary renal PNET were non-specific 17. The largest tumor dimension at diagnosis was 31 cm with central necrosis. This was also observed by Lee et al. that the most common image findings in PNET were the presence of a large necrotic and hemorrhagic mass along with extensive venous thrombosis 18.
We found that the majority (8/12) of the patients had tumors confined to the kidney. This is contrary to the present literature where most of the patients have a locally advanced or metastatic disease at presentation. Only four patients in our series had tumors extending locally beyond the kidney i.e capsular invasion, perinephric tissue invasion, lympho-vascular invasion, presence of renal vein and, or IVC thrombus. Our data revealed that none of the patient had metastasis at presentation. These results are at a disconcordance with previous reports showing metastasis in 40−65% of new patients with primary renal PNET 19,20. Most of the patients (83.3%) however, developed metastasis on follow-up.80% of the patients developed metastasis within 5 months. This high incidence of metastatic disease reflects the aggressive nature of this tumor.
In our study cohort majority of patients responded initially to surgery and adjuvant chemotherapy, but later had a significant rate of relapse (mainly local failure followed by distant metastasis). Only two patients in our study had no metastasis on follow up. The median metastasis free survival was 18.95 months. Local renal bed and lungs were the most common sites of metastasis observed in our study. Additionally, it revealed that despite patients showing an initial response to systemic chemotherapy, all but five of the 12 patients died of disease. Three patients received postoperative radiation in our study. Radiotherapy is currently recommended for unresectable tumors or unexpected positive margins 21 and may play a role in the local control of primary renal tumors with local extension beyond the kidney. Our results also signify the importance of early initiation of chemotherapy before the development of metastasis and suggest a potential role for radiation therapy in local control
Patients with non-metastatic tumors and a tumor thrombus in the renal vein or IVC developed metastasis to the lung within 6 months after nephrectomy, suggesting a potential benefit from using neo-adjuvant chemotherapy or initiation of systemic therapy soon after tumor resection. We report a high risk for recurrence/metastasis in patients with localized disease treated with nephrectomy alone 22,23 and poor outcomes in patients with metastatic disease 24. Similar to previous reports the outcome of our patients with metastatic tumors of the kidney was poor despite multimodal therapy, highlighting the need for novel treatment approaches for this very high-risk group25.
Our study was limited by its retrospective nature, the small number of cases, the incomplete data for some patients, the variation in therapies received over the extended study period, and the brief follow-up period for certain patients . The rarity of primary renal PNET makes a prospective study impractical and difficult.
Conclusion
As a rare renal tumor, renal PNET has a propensity to occur in young males. Due to the highly aggressive nature of the disease these patients have a very poor prognosis. Most patients either have distant metastasis at diagnosis or develop metastasis early on. Effective treatments are urgently needed. Apart from nephrectomy, adjuvant treatment with potent targeted anti-tumor agents is required for improved outcomes in disease management.
References
1. Potratz J, Dirksen U, Jürgens H, Craft A. Ewing sarcoma: clinical state-of-the-art. Pediatric hematology and oncology. 2012 Jan 27;29(1):1-1.
2. Applebaum MA, Worch J, Matthay KK, Goldsby R, Neuhaus J, West DC, DuBois SG. Clinical features and outcomes in patients with extraskeletal Ewing sarcoma. Cancer. 2011 Jul 1;117(13):3027-32.
3. Mubarak M, Kazi JI, Mohsin R, Hashmi A, Naqvi SA, ul Hassan Rizvi SA. Histopathology of surgically treated renal tumours in young adults: A developing country perspective. Journal of cancer research and clinical oncology. 2012 Feb;138:189-94.
4. TA S. Peripheral neuroectodermal tumors. Perspect Pediatr Pathol.. 1975;2:151-72.
5. Mukkunda R, Venkitaraman R, Thway K, Min T, Fisher C, Horwich A, Judson I. Primary adult renal Ewing's sarcoma: a rare entity. Sarcoma. 2009 Oct;2009.
6. Thyavihally YB, Tongaonkar HB, Gupta S, Kurkure PA, Amare P, Muckaden MA, Desai SB. Primitive neuroectodermal tumor of the kidney: a single institute series of 16 patients. Urology. 2008 Feb 1;71(2):292-6.
7. Risi E, Iacovelli R, Altavilla A, Alesini D, Palazzo A, Mosillo C, Trenta P, Cortesi E. Clinical and pathological features of primary neuroectodermal tumor/Ewing sarcoma of the kidney. Urology. 2013 Aug 1;82(2):382-6.
8. Jimenez RE, Folpe AL, Lapham RL, Ro JY, O'Shea PA, Weiss SW, Amin MB. Primary Ewing's
sarcoma/primitive neuroectodermal tumor of the kidney: a clinicopathologic and immunohistochemical analysis of 11 cases. The American journal of surgical pathology. 2002 Mar 1;26(3):320-7..
9. Ellison DA, Parham DM, Bridge J, Beckwith JB. Immunohistochemistry of primary malignant neuroepithelial tumors of the kidney: a potential source of confusion?: a study of 30 cases from the National Wilms Tumor Study Pathology Center. Human pathology. 2007 Feb 1;38(2):205-11.
10. Karpate A, Menon S, Basak R, Yuvaraja TB, Tongaonkar HB, Desai SB. Ewing sarcoma/primitive neuroectodermal tumor of the kidney: clinicopathologic analysis of 34 cases. Annals of Diagnostic Pathology. 2012 Aug 1;16(4):267-74.
11. Mohsin R, Hashmi A, Mubarak M, Sultan G, Shehzad A, Qayum A, Naqvi SA. Primitive neuroectodermal tumor/Ewing's sarcoma in adult uro-oncology: A case series from a developing country. Urology annals. 2011 May 1;3(2):103-7.
12. Teegavarapu PS, Rao P, Matrana MR, Cauley DH, Wood CG, Patel S, Tannir NM. Outcomes of adults with Ewing sarcoma family of tumors (ESFT) of the kidney: a single-institution experience. American journal of clinical oncology. 2017 Apr 1;40(2):189-93.
13. Murugan P, Rao P, Tamboli P, Czerniak B, Guo CC. Primary Ewing sarcoma/primitive neuroectodermal tumor of the kidney: a clinicopathologic study of 23 cases. Pathology & Oncology Research. 2018 Jan;24:153-9.
14. Kumar P, Singh A, Deshmukh A, Phulware RH, Rastogi S, Barwad A, Chandrashekhara SH, Singh V. Qualitative and quantitative CECT features for differentiating renal primitive neuroectodermal tumor from the renal cell carcinoma and its subtypes. The British Journal of Radiology. 2019 Feb 1;92(1094):20180738.
15. Hakky TS, Gonzalvo AA, Lockhart JL, Rodriguez AR. Primary Ewing sarcoma of the kidney: a symptomatic presentation and review of the literature. Therapeutic advances in urology. 2013 Jun;5(3):153-9.
16. Miller RW. Contrasting epidemiology of childhood osteosarcoma, Ewing’s tumor, and rhabdomyosarcoma. Natl Cancer Inst Monogr. 1981 Apr 1;56:9-15.
17. Higgins JC, Fitzgerald JM. Evaluation of incidental renal and adrenal masses. American family physician. 2001 Jan 15;63(2):288-95.
18. Lee H, Cho JY, Kim SH, Jung DC, Kim JK, Choi HJ. Imaging findings of primitive neuroectodermal tumors of the kidney. Journal of computer assisted tomography. 2009 Nov 1;33(6):882-6.
19. Rowe RG, Thomas DG, Schuetze SM, Hafez KS, Lawlor ER, Chugh R. Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology. 2013 Feb 1;81(2):347-53.
20. Zöllner S, Dirksen U, Jürgens H, Ranft A. Renal ewing tumors. Annals of oncology. 2013 Sep 1;24(9):2455-61.
21. DuBois SG, Krailo MD, Gebhardt MC, Donaldson SS, Marcus KJ, Dormans J, Shamberger RC, Sailer S, Nicholas RW, Healey JH, Tarbell NJ. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children's Oncology Group. Cancer. 2015 Feb 1;121(3):467-75.
22. Ohgaki K, Horiuchi K, Mizutani S, Sato M, Kondo Y. Primary Ewing’s sarcoma/primitive neuroectodermal tumor of the kidney that responded to low-dose chemotherapy with ifosfamide, etoposide, and doxorubicin. International journal of clinical oncology. 2010 Apr;15:210-4.
23. Alonso AH, Garate MM, Amo FH, Iribarren IM, Escudero RM, Sánchez JP, Jiménez JT, Fernandez CH. Primary renal Ewing's sarcoma. Archivos Espanoles de Urologia. 2011 Sep 1;64(7):636-9.
24. Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology. 2006 Aug 1;68(2):257- 62. 25. Ekram T, Elsayes KM, Cohan RH, Francis IR. Computed tomography and magnetic resonance features of renal Ewing sarcoma. Acta Radiologica. 2008 Nov;49(9):1085-90
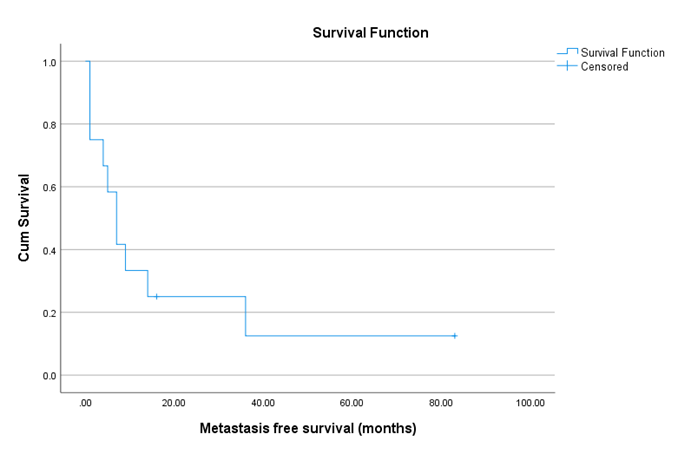
Figure 1
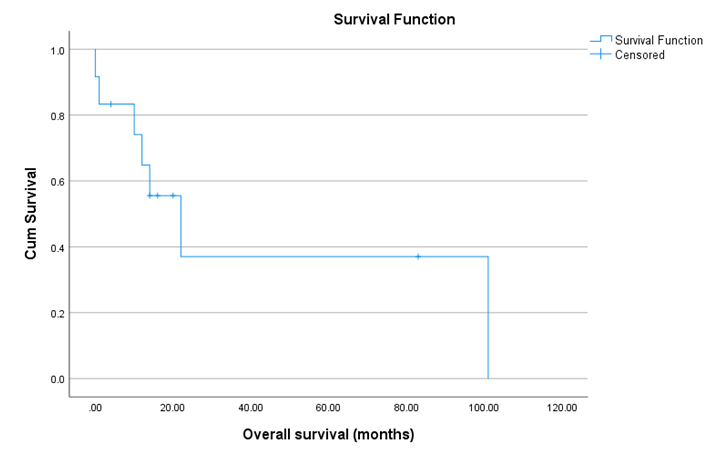
Figure 2
