18F-FDG PET Positive Castleman’s Disease of the Anterior Cervical Region in a 12 Year Old Girl: Case Report and Literature Review
18F-FDG PET Positive Castleman’s Disease of the Anterior Cervical Region in a 12 Year Old Girl: Case Report and Literature Review
Tiffany Seah1, Sophia Taylor2 Alan Ramsay3, Penny Shaw4, Stephen Daw5, Boo Messahel6*
1. Clinical School, University of Cambridge, UK.
2. Coppell High School, Texas, USA.
3. Department of Histopathology, University College Hospital London, UK.
4. Department of Radiology, University College Hospital London, UK.
5. Department of Paediatric and Adolescent Oncology, University College Hospital London, UK.
6. Department of Paediatric Oncology, Cambridge University Foundation Hospital, Cambridge, UK.
*Correspondence to: Boo Messahel, Department of Paediatric Oncology, Cambridge University Foundation Hospital, Cambridge, UK.
Copyright.
© 2024 Boo Messahel. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 10 July 2024
Published: 01 August 2024
Abstract
Introduction. Castleman’s disease is a non-malignant lymphoproliferative disorder generally involving the lymph nodes of young adults. Rarely, Castleman’s disease may present as a cervical mass in children.
Case. We report the case of a 12-year old girl who presented with a slow-growing, painless cervical mass. Diagnosis of hyaline vascular Castleman’s disease was made pre-operatively by histopathological examination of a core-needle biopsy specimen. Full-body 18F-FDG-PET/CT enabled exclusion of the multicentric form of disease. Surgical excision was curative and no evidence of recurrence was found at X months of follow-up.
Discussion. Castleman's disease presents a diagnostic challenge in neck region, as radiographic characteristics are often inconclusive and the differential is broad. The disease has unicentric and multicentric variants, with considerable differences in clinical characteristics, treatment and prognosis. We provide the most up-to-date literature review of paediatric patients with cervical Castleman’s disease, noting common presentations, diagnostic work-up employed and treatment outcomes.
Conclusion. Although rare, Castleman’s disease should be considered as a differential diagnosis in paediatric patients with a neck mass.
18F-FDG PET Positive Castleman’s Disease of the Anterior Cervical Region in a 12 Year Old Girl: Case Report and Literature Review
Introduction
Castleman’s disease (CD), a rare lymphoproliferative disease of uncertain aetiology, was first described in 1954 (1). The disease can occur anywhere in the lymphatic system, but is more commonly observed in the mediastinum, with a peak incidence at the fourth decade of life (Bonekamp, 2011). Neck involvement of CD is rare, and often manifests as an asymptomatic enlarging mass in the cervical region (2). CD is very uncommon in the paediatric population, with few reports of cervical CD in children (2).
Clinically, CD is always a diagnostic challenge because its symptoms and radiological findings are nonspecific. CD can be classified according to two systems, based on: 1) the dissemination of lymph node involvement, as unicentric Castleman disease (UCD) or multicentric Castleman disease (MCD) 2) histopathological features, as hyaline vascular type (HV), plasma cell type (PC), mixed type (Mix) or HHV-8 associated type (3).
In this paper, we report the rare case of a 12 year old girl with cervical CD. We furthermore present an up-to-date literature review of all known cases of paediatric cervical CD, comprising 50 patients from 1986 to 2016, to facilitate better understanding of the clinical features, imaging characteristics and treatment outcomes of this uncommon disease in children.
Case Report
A 12 year old girl, normally fit and well, was referred to our institution for further assessment after the discovery of a persistent left-sided cervical mass. The patient reported noticing the mass seven months prior to the appointment. The mass had gradually increased in size over 4 months with no further growth thereafter. She denied experiencing constitutional symptoms such as fever or night sweats suggestive of systemic illness.
On physical examination, a firm, mobile and non-tender mass measuring 3cm was noted in the left anterior base (level IV) of her neck. The skin overlying the mass was normal and no other masses were palpable in the neck.
Routine serum biochemistry and complete blood count data were all within normal limits. Virology screen including Hepatitis B and C, CMV, adenovirus and EBV were negative. Serologic investigation was negative for infectious mononucleosis, human immunodeficiency virus, rubella, cytomegalovirus and toxoplasmosis’ were negative’.
An ultrasound scan of the neck revealed a solitary enlarged lymph node in the anterior cervical chain, measuring 1.7x3.1x4.0 cm. It was hypoechoic and showed loss of normal architecture with small neighbouring reactive nodes. . The mass also showed prominent vascularity on Doppler. The thyroid, submandibular and parotid glands appeared normal on ultrasound.
Computer tomographic (CT) images of the neck with contrast medium enhancement showed an X by X cm well-defined mass in the left cervical anterior triangle. The lymph node in the left axilla on CT was described as a well-circumscribed, homogeneous mass lesion with moderate to intense enhancement and rapid washout
Given that radiological findings were suspicious for lymphoma, an ultrasound-guided Trucut biopsy of the mass was performed under a general anaesthetic to facilitate definitive diagnosis.
Histopathological examination of the lymph node revealed numerous follicles of varying sizes with markedly regressed and often vascularised/hyalinised germinal centres .Several follicles were also found to display the pathognomonic “lollipop lesions”, formed by the penetration of sclerotic blood vessels into the atrophic germinal centers. The mantle zones were expanded, with concentric layering of peripheral small lymphocytes resembling onion-skin. The intervening paracortex also showed marked vascular proliferation. Immunohistochemistry did not suggest a neoplastic process. CD20 emphasized both follicles and mantle zones with negative follicle staining for BCL2; CD21 emphasized the expanded follicular dendritic networks; and CD23 emphasized the mantle cell proliferation. CD3 and CD5 stained the prominent interfollicular T-cells.) CD123 showed frequent collections of plamacytoid dentritic cells and CD138 showed only occasional plasma cells.The node was negative for EBER and HHV8; and there was no evidence of monoclonality with kappa and lambda immunostains.
Together, these findings were consistent with a diagnosis of Castleman’s disease, hyaline vascular type.
Further full boding imaging was then undertaken to exclude multicentric involvement of the disease. This was done using an 18F-FDG PET/CT scan, which showed an enlarged solitary FDG-avid level IV cervical node measuring approximately 3.3cm x 1.28cm.
No other sites of increased FDG uptake either above or below the diaphragm were observed; and no focal lung or bony lesions were seen. This indicated that disease was localised, and a diagnosis of Unicentric Hyaline-Vascular Castleman’s disease was made. Surgical excision of the lesion was then performed.
The postoperative course was uneventful, clinical follow-ups were unremarkable, and there has been no evidence of recurrence at 7 years of follow up.
Discussion
Background
Castleman’s disease (CD) is an uncommon disorder caused by the benign proliferation of lymphoid tissue, which was first described by Castleman et al. in 1954 (1).
The aetiology of the disease remains uncertain. One theory suggests that the lesions in Castleman’s disease are caused by developmental growth disturbance of lymphoid tissue (4) Another hypothesis is that CD represents a reactive lymphoid hyperplasia, initiated by chronic antigenic stimulation associated with a viral trigger (5). Literature supporting the role of viral infection is mounting. Epidemiologic studies using polymerase chain reaction isolated the genome of HHV-8, most commonly in multicentric types of the disease (6, 7). Recently, Castleman’s disease has shown frequent association with human immunodeficiency virus infection and an increasing prevalence in HIV-infected individuals (8). However, in the cases reported in this review, no such associations were observed, Evidence supports interleukin-6 (IL-6) as an important player in the pathogenesis of CD. This crucial role of IL-6 is underscored by several observations. The symptomatology of the multicentric form of CD is directly correlated to serum levels of IL-6 (9). Surgical removal of all CD or the bulk of CD can lead to swift reductions in IL6 with rapid improvement in symptoms (9). Therapeutic interruption of the IL6 signaling cascade with anti-IL6 or -IL6R mAbs abrogates CD-related symptoms and, over time, leads to lymph node involution (10). Furthermore, human-IL6 transgenic mice develop a CD-like syndrome with plasmacytosis, splenomegaly, enlarged lymph nodes, fever, cachexia, and anemia, which can be ameliorated by administration of anti-IL6R mAbs (11, 12).
CD lesions can occur in any region of the body where lymphoid tissue is (13). In children, disease is most commonly found in the mediastinum (33%), followed by the abdomen (30%). The neck is considered a rare site for paediatric CD (14)..
Online medical journal databases were searched for patients aged 18 years or younger with cervical Castleman’s disease. After extensive review of the literature, to our knowledge, only 50 cases involving the paediatric neck have been reported between 1986 to 2016, including our own. The clinico-biological features of these children at presentation, treatment received and their outcomes are summarised in Table 1 (15-30).
Table 1. Patient presentation, treatment and outcome (Fig 1, 2)
Although CD can occur at any age, most literature supports a predisposition for the unicentric disease in young adulthood, with CD being rare in pediatric populations (14, 31-33). As reported in this literature review, the ages of children with cervical CD ranged from 1 to 18 years old (median:12 years old). Strikingly, 72% (36/50) of children presenting with cervical CD were between the ages of 10 and 18. Our patient, aged 12 years, thus falls in the most frequent age group. The disease is thought to affect both sexes equally (13), although in this review, the male to female ratio was approximately 1.2:1 (27 male: 23 female).
Of the 50 published cases, 26 cases classified the location of the cervical lump according to the system established by the Memorial Sloan Kettering Cancer Center (34). The most common location for a cervical CD in children appears to be Level II, representing 31% of lesions . Level IV was the least represented, with only 2 cases being reported to date, one of which was our patient. (Table 2)
Table 2. Distribution of Castleman’s disease in the paediatric neck
|
Location |
Number of patients |
Ratio |
|
Level I |
4 |
15% |
|
Level II |
8 |
31% |
|
Level III |
5 |
19% |
|
Level IV |
2 |
7% |
|
Level V |
7 |
27% |
|
Total |
26 |
100% |
Pathology
Pathologically, CD exhibits four different histological patterns (35). The hyaline vascular variant is most common(36), and is characterised by abnormal follicles with regressed germinal centers surrounded by expanded mantle zones comprised of small lymphocytes organised in concentric rings in an “onion-skin” arrangement. The regressed germinal center may become hyalinized and contain follicular dendritic cells (37). The plasma cell variant is characterised by hyperplastic germinal centers and sheets of mature polyclonal plasma cells in the interfollicular region(38). Marked vascular proliferation in the interfollicular region is present in both CD variants. Mixed forms demonstrate the presence of both hyaline vascular and plasma cell elements (39). The plasmablastic variant is less common and is associated with HIV infection. A subvariant of the plasma cell form, it is characterized by large plasmablasts harbouring human herpes virus (HHV)-8 and it can progress to frank plasmablastic monoclonal lymphoma (40).
Of the 50 children with cervical CD in this review, the majority, including our case, (48/50) had the hyaline-vascular variant, with 1 having the ‘mixed’ form (41) and 1 who did not report the histology (42).
Clinical presentation
Clinically the disease can be classified into two categories.
The unicentric variant of CD (UCD) is the most common form of the disease. By definition, it is confined to a single lymph-node chain or area and is usually of hyaline vascular histology (Bonekamp, 2014). All of the children evaluated in this review had unicentric cervical CD. A caveat to note would be that although no other sites of disease were found in these 50 children, only 9 received full-body work-ups to exclude possible multicentric disease. In the case of our patient, a full-body FDG-PET scan was performed to rule out other lesions. Patients with UCD are typically asymptomatic (43), a finding supported by our observation that of the 42 children with cervical CD whose presentation was described, 90% (38/42) presented asymptomatically or with painless enlargement of the mass, including our patient (Table 2). In two cases (44, 45), the cervical swelling may have compressed surrounding structures, causing patients to present with dysphagia and hoarseness. Rarely does UCD manifests with constitutional symptoms (46), although 3 cases in this review were noted to present with systemic unwellness involving fever and weight loss (30, 44, 47). No specific laboratory abnormalities were found in any of the patients evaluated in this analysis. This is consistent with the reported literature on unicentric CD (48).
The multicentric variant of CD (MCD) is a less common and more aggressive form. Its corresponding histological pattern is the plasma cell variant, and rarely the plasmablastic type. It is frequently accompanied by systemic manifestations such as fever, weight loss and diffuse lymphadenopathy (49). A number of laboratory abnormalities are seen, including anemia, thrombocytopenia, leukopenia, hypoalbuminemia, and hypergammaglobulinemia, and elevations in erythrocyte sedimentation rate (ESR) and interleukin (IL)-6 (39, 50) . MCD can progress to severe pancytopenia, multiorgan failure, and lymphoma (51). It can rarely be associated with polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes (POEMS) syndrome. Patients with MCD have an unfavourable prognosis (50).
Importantly, there has been documented record of multicentric disease in children involving the cervical lymph nodes (52-54). However, as cervical involvement was found as part of the work-up for their multicentric disease and not the primary complaint, these cases were excluded from this review which focuses on neck lumps in children which were diagnosed as CD.
Diagnosis
The diagnostic challenge that CD poses is related to the paucity of signs and symptoms in the unicentric variants, the absence of diagnostic specific markers and the ability of CD to mimic other neoplasms of the neck. In the pediatric population, this is especially true. Given the plethora of diseases that manifest as neck masses in children, CD is rarely on the differential and thus may be even harder to diagnose. Furthermore, given the nonspecific nature of this lymphoproliferative disease, it can often be confused with other lymphadenopathies (55). Across the cases in this analysis, the most highly represented differential diagnosis was lymphoma. In the case of our patient, lymphoma was also suspected until histopathological analysis proved otherwise. That CD had been labelled the “lymphoma imposter” by Denenberg and Levine (56) thus seems appropriate. Other diseases commonly confused with CD include infectious and inflammatory lesions such as lymphadenitis, tuberculosis, sarcoidosis, toxoplasmosis, human immunodeficiency virus, CMV, mononucleosis, cat scratch disease, branchial cleft cyst, and hemangioma (32).
CD is also characteristically difficult to diagnose on imaging examinations because of its lack of unique radiographic features (46, 57). In this review, the main imaging modalities used in the work-up for cervical masses in children were: computed tomography 23/50 (CT) , ultrasound (16/50) and magnetic resonance imaging (8/50)(MRI). However, all three modalities are often nonspecific and definitive diagnosis by histopathologic examination is required.
The typical ultrasonographic finding described in the literature is a uniformly hypoechoic mass with good acoustic enhancement, an observation consonant with our present case and those in the review. Wagner et al recently suggested that findings characteristic for CD may be present on Colour Doppler Ultrasound (CDU), such as prominent peripheral vascular proliferation in the node. This is not seen in healthy or reactive lymph nodes which tend to preserve a normal vascularity pattern with central hilar vessels, and is absent from malignant lymph nodes which typically present with a mixed vascular distribution including both central and peripheral flow (58)
CT scan with contrast of CD shows a well-circumscribed, homogenously enhancing mass, with the hyaline vascular variant enhancing more than the plasma cell variant due to its greater vascularity (Bonekamp, 2011). It has been suggested that the presence of a non-enhanced stellar scar within the center of the mass, due to dense fibrous tissue, could be a diagnostic clue for CD(59). Magnetic resonance imaging (MRI) can be employed to better define the extent of surrounding soft tissue involvement. The classic finding is that of a homogeneous, isointense to slightly hyperintense T1-weighted signal and high signal intensity on T2-weighted imaging (46).
In contrast to the above conventional imaging techniques, 18F-FDG-PET/CT is a hybrid diagnostic technique that allows information regarding both morphological image and metabolic function to be acquired, enabling a dual gain in diagnostic accuracy (60). An increase in glucose metabolic activity, reflected by radiotracer accumulation, can be found in both inflammatory and malignant lesions. As such, lymph nodes involved in CD are well-visualised as areas of increased 18F-FDG avidity on PET/CT(61).
To our knowledge, our present patient is the third reported case for which FDG-PET was used in the diagnostic work-up for CD in children (Toita and Elfintherou). Compared to conventional imaging techniques, FDG-PET offers several advantages in the evaluation of CD. 18F-FDG PET/CT has been shown to be more sensitive than contrast-enhanced CT, as it is able to visualise disease in non-enlarged lymph nodes which may be overlooked on CT (62, 63). This is important for the determination of disease multicentricity, which has therapeutic implications. Alternatively, it avoids mistaking reactive lymph nodes with increased size on CT as pathological(60). Furthermore, a PET study can be used to monitor disease response to treatment, as evidenced by a decrease in metabolic activity (60).
Murphy et al proposed that FDG-PET might even provide a means of differentiating benign CD lesions from malignant lymphoma, based on the finding that lymph nodes in CD accumulated FDG in a range lower than that seen for low-and intermediate-grade lymphomas (64). In line with this, several other authors (65, 66) have similarly reported low Standardised Uptake Values (SUVmax) for FDG-avid lymph nodes in CD. SUVmax for our patient was 3.5, supporting Murphy’s hypothesis. On the other hand, others (67, 68) have found high FDG avidity in their cases of CD, leading to the opinion that lymphoma and CD share too many similarities on PET imaging, such as pattern of lymphatic involvement, extra-nodal sites, and overlap in SUVmax values, to be reliably differentiated.
A study that may reconcile these conflicting findings, is that by Lee et al, who reported that FDG uptake in CD was correlated with disease multicentricity and clinical manifestation (61). Unicentric asymptomatic CD had SUVmax in the lower range (3.3 ± 1.1), but multicentric symptomatic CD had significantly higher SUVmax (7.0 ± 4.6). A large study of non-Hodgkin’s Lymphoma by Schöder et al described SUVmax in the ranges of 7.0 ± 3.1 for indolent lymphoma and 19.6 ± 9.3 for aggressive lymphoma (69). Taken together, this suggests that while FDG-PET may have a role in differentiating asymptomatic UCD from most lymphomas, this distinction is blurred in the presence of symptomatic MCD, which may exhibit higher FDG avidity comparable to less aggressive lymphomas (69). A better understanding of the role of FDG-PET/CT in differentiating CD from lymphoma requires evaluation with large sample size studies.
Ultimately regardless of imaging findings, biopsy remains the standard procedure for establishing an unequivocal diagnosis, as it enables examination for the cytological and architectural changes that typify CD (70).
Treatment
Definitive treatment of unicentric disease involves complete surgical excision of the affected node, with excellent prognosis (71). In support of this, our review showed that 47 children with cervical UCD received complete resection as therapy and no tumour recurrence was noted during the follow-up period (mean follow up: 38 months). Zhang et al reported no recurrence after 136 months of follow-up in one patient, which represents the longest follow-up of paediatric cervical CD to our knowledge (52). In our case, no signs of recurrence were identified in our patient 7 years after excision of the mass. Rarely, recurrence of localised disease has been reported after incomplete surgical removal (72).
For patients with UCD not amenable to complete resection, radiation therapy has achieved favourable outcomes (73). To our knowledge, there has only been one reported case of a child with unresectable cervical UCD due to involvement of major vascular structures causing life-threatening bleeding during attempted surgical excision (74). While trial with steroids and vinblastine showed no response, delivery of local radiotherapy was able to achieve complete radiographic and clinical resolution of disease, with no recurrence at 12 months follow-up.
In contrast, due to the rarity and heterogeneity of multicentric CD, randomised clinical trials remain a challenge, and there is no established standard of care for treatment. Treatment options for MCD are mostly based on few nonrandomised prospective studies, small case series and expert opinion; as such, the body of evidence must be interpreted cautiously, bearing in mind non-uniformity of response criteria and heterogenetity of patient population (75)
Three main treatment strategies have been employed based on present understanding of MCD: 1) anti-inflammatory and immunosuppressive therapies 2) cytotoxic elimination of cells responsible for hypercytokinemia 3) blockade of IL-6 signaling (76)
The use of corticosteroids to control the intense inflammation associated with multicentric disease is common, but most patients relapse during steroid tapering (77, 78). Interferon-α is being increasingly employed as a treatment option, particularly in HIV positive patients, due to its antiviral and immunomodulatory properties (79-81). Other immunosuppressive therapies being explored include Cyclosporin A for HIV negative MCD (82). Rituximab, a monoclonal chimeric antibody that targets CD20 on B cells, is well established as effective first-line therapy in HIV-associated CD (83-85). However, it has not been found to achieve durable response in HIV-negative disease (86, 87).
Cytotoxic chemotherapy regimens for MCD have been modelled on those used for treatment of lymphoma, such as cyclophosphamide, vincristine, doxorubicin and either prednisone (CHOP) or dexamethasone (CVAD). Although able to induce responses in many MCD patients by eradicating a large portion of hypercytokine-secreting cells, complete remission rates are considerable lower and patients commonly progress or experience infectious toxicities (72, 73).
Although not completely understood, the pathophysiology of CD provides opportunities for therapeutic intervention, with evidence accumulating for a major role for Interleukin-6 (IL-6) in disease pathogenesis. The strong rationale for the targeting of IL-6 has led to the development of two agents: siltuximab and tocilizumab, monoclonal antibodies targeting IL-6 and its receptor (IL-6R), respectively. Siltuximab has recently been approved by the US Food and Drug Administration (FDA) for the treatment of patients with HIV-negative, HHV-8-negative MCD, where it has been shown to alleviate IL-6-dependent systemic symptoms and reverse laboratory abnormalities (88). Tocilizumab has been approved to treat MCD in Japan, and has demonstrated effectiveness at inducing and maintaining remission (89). Importantly, while both mAbs are potential candidates for frontline therapy in MCD, they require lifelong administration, and are not effective in all cases of MCD, possibly due to disease heterogeneity or misdiagnosis, amidst numerous other variables (76).
Recently, therapeutic approaches targeting pathways upstream of IL-6 have been reported for treatment of MCD. Bortezomib, a novel proteasome inhibitor that preferentially targets plasma cells, has been reported to induce remission in 4 cases of MCD (90, 91). It has been suggested that Bortezomib exerts its clinical activity through lowering of IL-6 levels via disruption of NF-κB signaling cascade (92). Thalidomide, an immunomodulator able to decrease IL-6 levels, has also demonstrated effectiveness in achieving partial or complete response in four patients with MCD to date (93, 94). Anakinra, an IL-1 receptor antagonist, has also been reported to induce remission in a pediatric patient with MCD refractory to both chemotherapy and rituximab, and in an adult patient who did not respond to anti-IL-6 therapy (95, 96). Its mechanism of action is by blocking of the IL1 receptor which inhibits the NKκB pathway, thus reducing the transcription of pro-inflammatory cytokines, including IL-6. Despite therapy, the prognosis of multicentric disease is poor, with a median survival range of 14-30 months. The main cause of mortality appears to be infections and sepsis, multiorgan failures and secondary malignancies (39). Further elucidation of the molecular aberrations driving MCD is needed to provide additional candidate targeted therapies in the future.
Conclusion
In conclusion, cervical UCD in children is rare, with only 50 cases described to date. As this review demonstrates, doctors should be aware of CD when presented with complaints of a painless, enlarging neck mass, especially in the paediatric population. The diagnosis of this entity is a clinical challenge as symptoms and imaging characteristics are often nonspecific. FDG-PET is of use in assessing for multicentric disease. Surgical excision is ultimately diagnostic and curative for UCD, although treatment options for MCD remain controversial. The clinical presentation, histopathological/radiological investigations and treatment outcome of our case were concordant with previous reports in the literature for paediatric cervical UCD. In light of the absence of specific diagnostic criteria for differentiating CD from numerous other etiologies of neck lumps in children, further research is needed for the development of more sensitive diagnostic methods.
References
1. Castleman B, Towne VW. Case records of the Massachusetts General Hospital; weekly clinicopathological exercises; founded by Richard C. Cabot. N Engl J Med. 1954;251(10):396-400.
2. Rabinowitz MR, Levi J, Conard K, Shah UK. Castleman disease in the pediatric neck: a literature review. Otolaryngol Head Neck Surg. 2013;148(6):1028-36.
3. Lin CY, Chang YL. Castleman's disease in the head and neck region: Meta-analysis of reported cases in Taiwan and literature review. J Formos Med Assoc. 2010;109(12):913-20.
4. Zheng X, Pan K, Cheng J, Dong L, Yang K, Wu E. Localized Castleman disease in retroperitoneum: newly discovered features by multi-detector helical CT. Abdom Imaging. 2008;33(4):489-92.
5. Singletary LA, Karcnik TJ, Abujudeh H. Hyaline vascular-type Castleman disease: a rare cause of a hypervascular retroperitoneal mass. Abdom Imaging. 2000;25(2):207-9.
6. Tohda S, Murakami N, Nara N. Human herpesvirus 8 DNA in HIV-negative Japanese patients with multicentric Castleman's disease and related diseases. Int J Mol Med. 2001;8(5):549-51.
7. Menke DM, Chadbum A, Cesarman E, Green E, Berenson J, Said J, et al. Analysis of the human herpesvirus 8 (HHV-8) genome and HHV-8 vIL-6 expression in archival cases of castleman disease at low risk for HIV infection. Am J Clin Pathol. 2002;117(2):268-75.
8. Mylona EE, Baraboutis IG, Lekakis LJ, Georgiou O, Papastamopoulos V, Skoutelis A. Multicentric Castleman's disease in HIV infection: a systematic review of the literature. AIDS Rev. 2008;10(1):25-35.
9. Yoshizaki K, Matsuda T, Nishimoto N, Kuritani T, Taeho L, Aozasa K, et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74(4):1360-7.
10. Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, et al. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95(1):56-61.
11. Brandt SJ, Bodine DM, Dunbar CE, Nienhuis AW. Dysregulated interleukin 6 expression produces a syndrome resembling Castleman's disease in mice. J Clin Invest. 1990;86(2):592-9.
12. Gaba AR, Stein RS, Sweet DL, Variakojis D. Multicentric giant lymph node hyperplasia. Am J Clin Pathol. 1978;69(1):86-90.
13. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. 1972;29(3):670-83.
14. Zhong LP, Chen GF, Zhao SF. Cervical Castleman disease in children. Br J Oral Maxillofac Surg. 2004;42(1):69-71.
15. Wolf M, Kessler A, Horovitz A. Benign angiofollicular lymph node hyperplasia (Castleman's disease) presenting as a solitary cervical mass. J Oral Maxillofac Surg. 1991;49(10):1129-31.
16. Salisbury JR. Castleman's disease in childhood and adolescence: report of a case and review of literature. Pediatr Pathol. 1990;10(4):609-15.
17. Glazer M, Rao VM, Reiter D, McCue P. Isolated Castleman disease of the neck: MR findings. AJNR Am J Neuroradiol. 1995;16(4):669-71.
18. Yi AY, deTar M, Becker TS, Rice DH. Giant lymph node hyperplasia of the head and neck (Castleman's disease): a report of five cases. Otolaryngol Head Neck Surg. 1995;113(4):462-6.
19. Tuerlinckx D, Bodart E, Delos M, Remacle M, Ninane J. Unifocal cervical Castleman disease in two children. Eur J Pediatr. 1997;156(9):701-3.
20. Patel U, Forte V, Taylor G, Sirkin W. Castleman's disease as a rare cause of a neck mass in a child. J Otolaryngol. 1998;27(3):171-3.
21. Parez N, Bader-Meunier B, Roy CC, Dommergues JP. Paediatric Castleman disease: report of seven cases and review of the literature. Eur J Pediatr. 1999;158(8):631-7.
22. Bond SE, Saeed NR, Palka I, Carls FP. Castleman's disease presenting as a midline neck mass. Br J Plast Surg. 2003;56(1):62-4.
23. Chen WC, Jones D, Ho CL, Cheng CN, Tseng JY, Tsai HP, et al. Cytogenetic anomalies in hyaline vascular Castleman disease: report of two cases with reappraisal of histogenesis. Cancer Genet Cytogenet. 2006;164(2):110-7.
24. Chen CH, Liu HC, Tung KY, Lee JJ, Liu CL, Liu TP. Surgical outcome of superficial and deep Castleman disease. ANZ J Surg. 2007;77(5):339-43.
25. Souza KC, Silva SJ, Salomao E, Silva AM, Faria PR, Queiroz LF, et al. Cervical Castleman's disease in childhood. J Oral Maxillofac Surg. 2008;66(5):1067-72.
26. Zhong LP, Wang LZ, Ji T, Hu YH, Hu YJ, Ye WM, et al. Clinical analysis of Castleman disease (hyaline vascular type) in parotid and neck region. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(3):432-40.
27. Bouguila J, Lahmer I, Abdelkefi M, Affissath A, Trimeche M, Boughammoura L. Cervical unicentric Castleman's disease in children. Eur Ann Otorhinolaryngol Head Neck Dis. 2013;130(4):221-3.
28. Zawawi F, Varshney R, Haegert DG, Daniel SJ. Castleman's Disease: a rare finding in a pediatric neck. Int J Pediatr Otorhinolaryngol. 2014;78(2):370-2.
29. Melkundi RS, Prasad KC, Jalisatgi RR, Swami G, Karunasagar A. Unicentric castlemans disease: unusual disorder of the neck a case review. J Clin Diagn Res. 2015;9(4):Md03-4.
30. R RS, Ashish R, V KC. Castlemans's disease: an unusual presentation in cervical region. Indian Pediatr. 2001;38(4):419-22.
31. Penfold CN, Cottrell BJ, Talbot R. Neonatal giant lymph node hyperplasia (Castleman's disease) presenting in the head and neck. Br J Oral Maxillofac Surg. 1991;29(2):110-1.
32. Song JJ, Jung MH, Woo JS, Chae SW, Hwang SJ, Lee HM. Castleman's disease of the head and neck. Eur Arch Otorhinolaryngol. 2006;263(2):160-3.
33. Park JH, Lee SW, Koh YW. Castleman disease of the parotid gland in childhood: an unusual entity. Auris Nasus Larynx. 2008;35(3):451-4.
34. Ferlito A, Robbins KT, Silver CE, Hasegawa Y, Rinaldo A. Classification of neck dissections: an evolving system. Auris Nasus Larynx. 2009;36(2):127-34.
35. Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16(4):236-46.
36. Dispenzieri A, Armitage JO, Loe MJ, Geyer SM, Allred J, Camoriano JK, et al. The clinical spectrum of Castleman's disease. Am J Hematol. 2012;87(11):997-1002.
37. Cakabay B, Akbulut S, Sezgin A, Gomceli I, Arikok AT, Akgul Ozmen C, et al. Castleman's disease as cervical mass: a report of three cases and review of the literature. G Chir. 2009;30(8-9):335-8.
38. Sanchez-Cuellar A, de Pedro M, Martin-Granizo R, Berguer A. Castleman disease (giant lymph node hyperplasia) in the maxillofacial region: a report of 3 cases. J Oral Maxillofac Surg. 2001;59(2):228-31.
39. Casper C. The aetiology and management of Castleman disease at 50 years: translating pathophysiology to patient care. Br J Haematol. 2005;129(1):3-17.
40. Dupin N, Diss TL, Kellam P, Tulliez M, Du MQ, Sicard D, et al. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood. 2000;95(4):1406-12.
41. Minerva T, Franza R, Panico D, Pizzolante M, Cambo M. Castleman's disease with diffuse cervical localisation: case report. Acta Otorhinolaryngol Ital. 2004;24(4):234-8.
42. Rao HG, Street I, Capper R. Unusual location for Castleman's disease. J Laryngol Otol. 2009;123(1):e3.
43. Raut V, Cullen J, Hughes D. Giant lymph node hyperplasia a diagnostic dilemma in the neck. Auris Nasus Larynx. 2001;28(2):185-8.
44. Koslin DB, Berland LL, Sekar BC. Cervical Castleman disease: CT study with angiographic correlation. Radiology. 1986;160(1):213-4.
45. Dangoor E, Elidan J, Gomori JM, Dano I. Castleman's disease of the retropharynx: a benign tumor in a 12-year-old girl. Am J Otolaryngol. 1998;19(3):194-7.
46. Bonekamp D, Horton KM, Hruban RH, Fishman EK. Castleman disease: the great mimic. Radiographics. 2011;31(6):1793-807.
47. Kansara AH, Mehta SP. An exrathoracic presentation of castleman's disease. Indian J Otolaryngol Head Neck Surg. 2005;57(2):166-7.
48. Chen YF, Zhang WD, Sun CZ, Ouyang D, Chen WK, Luo RZ, et al. Clinical features and outcomes of head and neck castleman disease. J Oral Maxillofac Surg. 2012;70(10):2466-79.
49. Ide M, Kawachi Y, Izumi Y, Kasagi K, Ogino T. Long-term remission in HIV-negative patients with multicentric Castleman's disease using rituximab. Eur J Haematol. 2006;76(2):119-23.
50. Dham A, Peterson BA. Castleman disease. Curr Opin Hematol. 2007;14(4):354-9.
51. Waterston A, Bower M. Fifty years of multicentric Castleman's disease. Acta Oncol. 2004;43(8):698-704.
52. Zhang J, Li C, Lv L, Yang C, Kong XR, Zhu J, et al. Clinical and experimental study of Castleman disease in children. Pediatr Blood Cancer. 2015;62(1):109-14.
53. Drolet JP, Lefebvre MA, Bernard C, Mitchell D, McCusker C, Mazer B. Multicentric castleman disease in a child with primary immunodeficiency. Pediatr Blood Cancer. 2010;55(6):1198-200.
54. Turcotte LM, Correll CK, Reed RC, Moertel CL. Sustained remission of severe Multicentric Castleman disease following multiagent chemotherapy and tocilizumab maintenance. Pediatr Blood Cancer. 2014;61(4):737-9.
55. Ramsay AD. Reactive lymph nodes in pediatric practice. Am J Clin Pathol. 2004;122 Suppl:S87-97.
56. Denenberg S, Levine PA. Castleman's disease--the lymphoma impostor. Laryngoscope. 1984;94(5 Pt 1):601-4.
57. McAdams HP, Rosado-de-Christenson M, Fishback NF, Templeton PA. Castleman disease of the thorax: radiologic features with clinical and histopathologic correlation. Radiology. 1998;209(1):221-8.
58. Wagner N, Maden Z. An unusual unifocal presentation of Castleman's disease in a young woman with a detailed description of sonographic findings to reduce diagnostic uncertainty: a case report. BMC Res Notes. 2013;6:97.
59. Tan TY, Pang KP, Goh HK, Teo EL, Abhilash B, Walford N. Castleman's disease of the neck: a description of four cases on contrast-enhanced CT. Br J Radiol. 2004;77(915):253-6.
60. Pelosi E, Skanjeti A, Cistaro A, Arena V. Fluorodeoxyglucose-positron emission tomography/computed tomography in the staging and evaluation of treatment response in a patient with Castleman's disease: a case report. J Med Case Rep. 2008;2:99.
61. Lee ES, Paeng JC, Park CM, Chang W, Lee WW, Kang KW, et al. Metabolic characteristics of Castleman disease on 18F-FDG PET in relation to clinical implication. Clin Nucl Med. 2013;38(5):339-42.
62. Barker R, Kazmi F, Stebbing J, Ngan S, Chinn R, Nelson M, et al. FDG-PET/CT imaging in the management of HIV-associated multicentric Castleman's disease. Eur J Nucl Med Mol Imaging. 2009;36(4):648-52.
63. Toita N, Kawamura N, Hatano N, Takezaki S, Ohkura Y, Yamada M, et al. A 5-year-old boy with unicentric Castleman disease affecting the mesentery: utility of serum IL-6 level and (18)F-FDG PET for diagnosis. J Pediatr Hematol Oncol. 2009;31(9):693-5.
64. Murphy SP, Nathan MA, Karwal MW. FDG-PET appearance of pelvic Castleman's disease. J Nucl Med. 1997;38(8):1211-2.
65. Ding Q, Zhang J, Yang L. (18)F-FDG PET/CT in multicentric Castleman disease: a case report. Ann Transl Med. 2016;4(3):58.
66. Blockmans D, Maes A, Stroobants S, Bobbaers H, Mortelmans L. FDG positron emission tomographic scintigraphy can reveal Castleman's disease as a cause of inflammation. Clin Nucl Med. 2001;26(11):975-6.
67. Puranik AD, Purandare NC, Shah S, Agrawal A, Rangarajan V. Multicentric Castleman's disease: Closest mimic of lymphoma on FDG PET/CT. Indian J Nucl Med. 2013;28(2):124-5.
68. Elboga U, Narin Y, Urhan M, Sahin E. FDG PET/CT appearance of multicentric Castleman's disease mimicking lymphoma. Rev Esp Med Nucl Imagen Mol. 2012;31(3):142-4.
69. Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(21):4643-51.
70. Madan R, Chen JH, Trotman-Dickenson B, Jacobson F, Hunsaker A. The spectrum of Castleman's disease: mimics, radiologic pathologic correlation and role of imaging in patient management. Eur J Radiol. 2012;81(1):123-31.
71. Lin CY, Huang TC. Cervical posterior triangle castleman's disease in a child - case report & literature review. Chang Gung Med J. 2011;34(4):435-9.
72. Herrada J, Cabanillas F, Rice L, Manning J, Pugh W. The clinical behavior of localized and multicentric Castleman disease. Ann Intern Med. 1998;128(8):657-62.
73. Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670-6.
74. Farruggia P, Trizzino A, Scibetta N, Cecchetto G, Guerrieri P, D'Amore ES, et al. Castleman's disease in childhood: report of three cases and review of the literature. Ital J Pediatr. 2011;37:50.
75. El-Osta HE, Kurzrock R. Castleman's disease: from basic mechanisms to molecular therapeutics. Oncologist. 2011;16(4):497-511.
76. Fajgenbaum DC, Kurzrock R. Siltuximab: a targeted therapy for idiopathic multicentric Castleman disease. Immunotherapy. 2016;8(1):17-26.
77. Frizzera G. Castleman's disease and related disorders. Semin Diagn Pathol. 1988;5(4):346-64.
78. Tsukamoto Y, Hanada N, Nomura Y, Hiki Y, Kasai K, Shigematsu H, et al. Rapidly progressive renal failure associated with angiofollicular lymph node hyperplasia. Am J Nephrol. 1991;11(5):430-6.
79. Kumari P, Schechter GP, Saini N, Benator DA. Successful treatment of human immunodeficiency virus-related Castleman's disease with interferon-alpha. Clin Infect Dis. 2000;31(2):602-4.
80. Nord JA, Karter D. Low dose interferon-alpha therapy for HIV-associated multicentric Castleman's disease. Int J STD AIDS. 2003;14(1):61-2.
81. Oksenhendler E, Duarte M, Soulier J, Cacoub P, Welker Y, Cadranel J, et al. Multicentric Castleman's disease in HIV infection: a clinical and pathological study of 20 patients. Aids. 1996;10(1):61-7.
82. Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A : a case report. J Clin Exp Hematop. 2013;53(1):95-9.
83. Gerard L, Berezne A, Galicier L, Meignin V, Obadia M, De Castro N, et al. Prospective study of rituximab in chemotherapy-dependent human immunodeficiency virus associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial. J Clin Oncol. 2007;25(22):3350-6.
84. Bower M, Powles T, Williams S, Davis TN, Atkins M, Montoto S, et al. Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836-9.
85. Bower M, Newsom-Davis T, Naresh K, Merchant S, Lee B, Gazzard B, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman's Disease. J Clin Oncol. 2011;29(18):2481-6.
86. Gholam D, Vantelon JM, Al-Jijakli A, Bourhis JH. A case of multicentric Castleman's disease associated with advanced systemic amyloidosis treated with chemotherapy and anti-CD20 monoclonal antibody. Ann Hematol. 2003;82(12):766-8.
87. Ocio EM, Sanchez-Guijo FM, Diez-Campelo M, Castilla C, Blanco OJ, Caballero D, et al. Efficacy of rituximab in an aggressive form of multicentric Castleman disease associated with immune phenomena. Am J Hematol. 2005;78(4):302-5.
88. Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res. 2013;19(13):3659-70.
89. Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627-32.
90. Hess G, Wagner V, Kreft A, Heussel CP, Huber C. Effects of bortezomib on pro-inflammatory cytokine levels and transfusion dependency in a patient with multicentric Castleman disease. Br J Haematol. 134. England2006. p. 544-5.
91. Sobas MA, Alonso Vence N, Diaz Arias J, Bendana Lopez A, Fraga Rodriguez M, Bello Lopez JL. Efficacy of bortezomib in refractory form of multicentric Castleman disease associated to poems syndrome (MCD-POEMS variant). Ann Hematol. 2010;89(2):217-9.
92. Juvekar A, Manna S, Ramaswami S, Chang TP, Vu HY, Ghosh CC, et al. Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB--dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9(2):183-94.
93. Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326-35.
94. Starkey CR, Joste NE, Lee FC. Near-total resolution of multicentric Castleman disease by prolonged treatment with thalidomide. Am J Hematol. 2006;81(4):303-4.
95. El-Osta H, Janku F, Kurzrock R. Successful treatment of Castleman's disease with interleukin-1 receptor antagonist (Anakinra). Mol Cancer Ther. 2010;9(6):1485-8.
96. Galeotti C, Tran TA, Franchi-Abella S, Fabre M, Pariente D, Kone-Paut I. IL-1RA agonist (anakinra) in the treatment of multifocal castleman disease: case report. J Pediatr Hematol Oncol. 2008;30(12):920-4.
97. G Yildirim, G Berkiten, K Turkoz. Castleman’s Disease In Childhood. The Internet Journal of Otorhinolaryngology. 2008 Volume 10 Number 2
98. Vianna PM, Pastore PG, Cristofani L, et al. Castleman disease: an uncommon diagnosis in pediatrics. Autopsy Case Rep [Internet]. 2012; 2(3):39-44.
99. Ashok S. Komaranchath, A.H. Rudresh, Kuntegowdenahalli Lakshmaiah C., Chennagiri S. Premalata, Dasappa Loknatha, Linu A. Jacob. Unicentric Castleman’s disease: An unusual cause of neck swelling J Case Rep Images Oncology 2015;1:5–9.
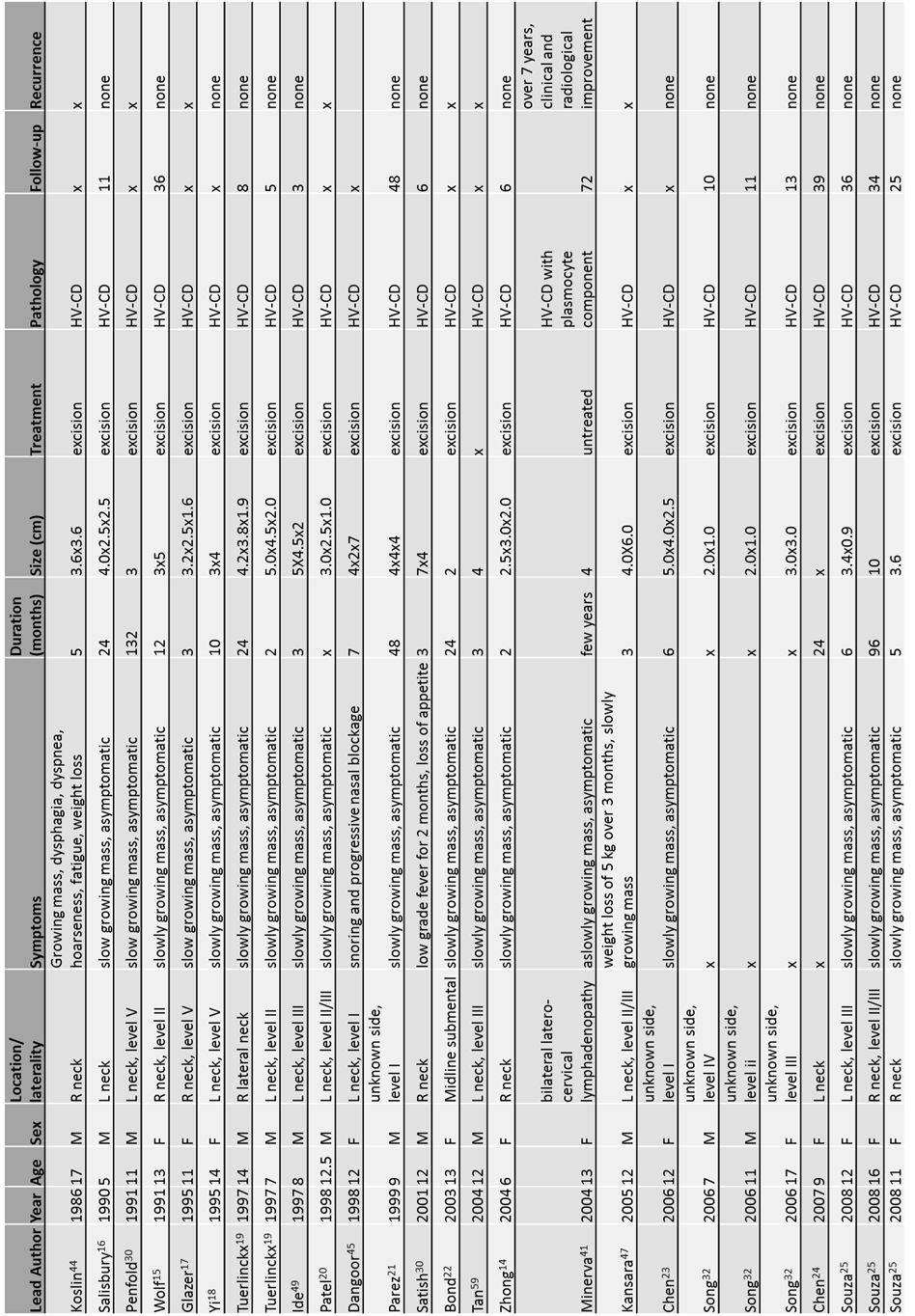
Figure 1
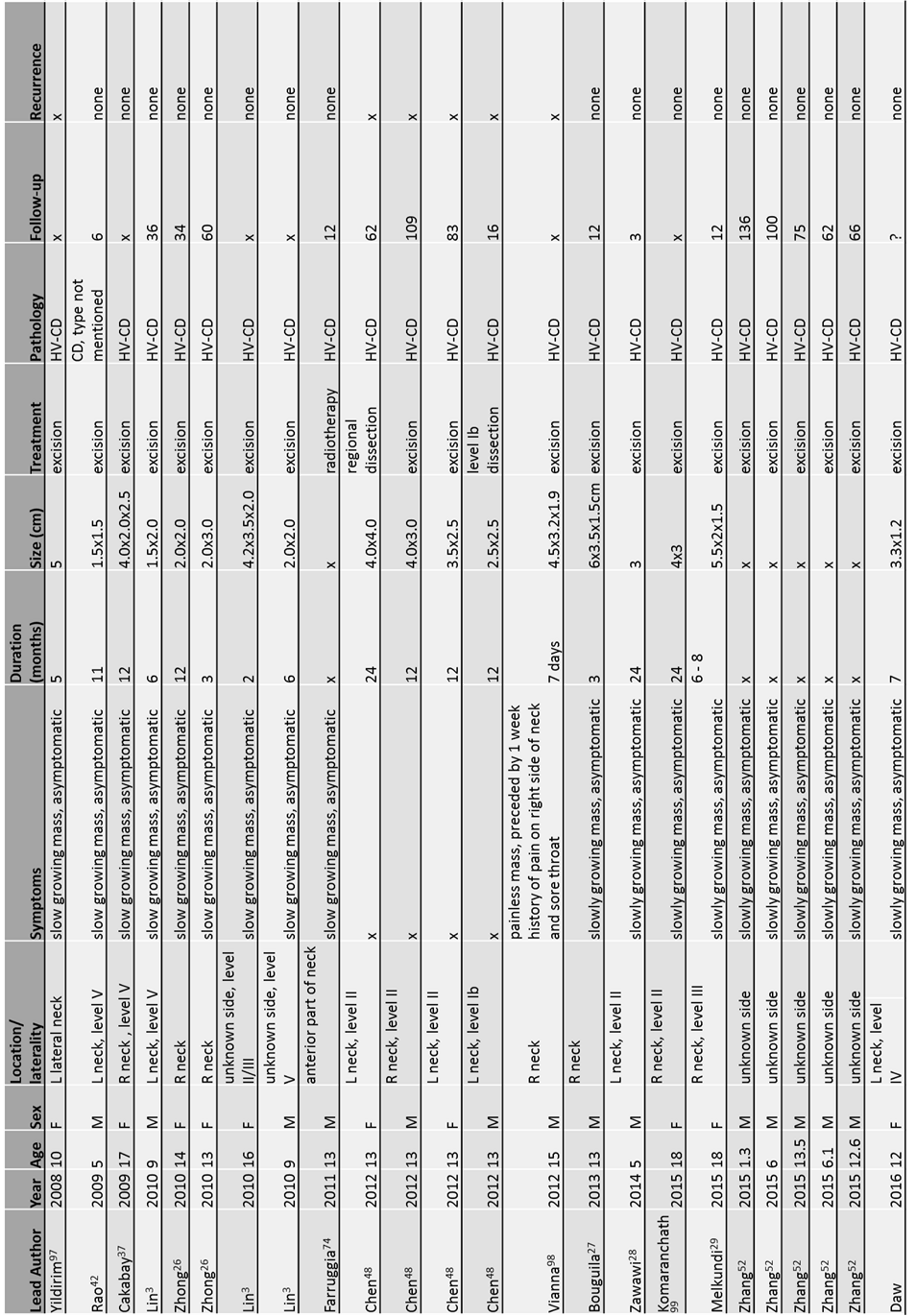
Figure 2
