A Comprehensive Approach to Diagnosing and Treating Glioblastoma
A Comprehensive Approach to Diagnosing and Treating Glioblastoma
Mohan Babu*
*Correspondence to: Mohan Babu, India.
Copyright.
© 2024 Mohan Babu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 30 August 2024
Published: 02 September 2024
Abstract
Glioblastoma is the most common type of brain cancer in adults. Current treatment is based on surgery, radiotherapy, and antibiotics. Despite advances in our understanding of glioblastoma pathogenesis, we still face increased morbidity, changes in quality of life, adverse events, recurrences, and a median overall survival of 15 months. Over the past few years, our understanding of glioblastoma pathophysiology has advanced rapidly, leading to important insights and new therapeutic ideas. In this review, we present the clinical outcomes, current standards of care, and outcomes of patients diagnosed with glioblastoma from R-based primary data analysis. We also present the latest developments and theories regarding the pathophysiological basis and new treatments such as cancer vaccines and personalized therapies.
Keywords: glioblastoma; molecular pathology; omics; pathogenesis; personalized therapies
A Comprehensive Approach to Diagnosing and Treating Glioblastoma
Introduction
Glioblastoma is the most common type of brain tumor in adults (1). The incidence of glioblastoma has increased over the past 20 years due to improved public expectations and access to more accurate diagnostic tools such as MRI [2–4]. In a recent article based on a prospective registry of central nervous system (CNS) tumors in the United States [5], glioblastoma accounted for 54% of brain gliomas, with an annual incidence of 3.19 per 100,000 people. The peak incidence of isocitrate dehydrogenase (IDH) wild-type glioblastoma occurs around the age of 6 years, whereas the peak incidence of IDH mutant glioblastoma occurs earlier, at approximately 4–5 years [6]. Despite numerous clinical trials [7–9] and treatment of these tumors, median survival is poor, approximately 14 to 20 months, with a five-year survival rate of 5%, depending on age at diagnosis, molecular features, and treatment [10]. Due to the poor outcome of this disease, a better understanding of the pathogenesis and metabolic processes of glioblastoma is necessary. Over the years, advances in many areas have led to new insights and new therapeutic approaches. Precision medicine has provided a new approach to many tumor diseases thanks to the rise of omics technology [11,12]. Its main goal is to develop a personalized perspective on the disease, including differentiation by the patient's condition and preferences. This approach requires the collection of all glioblastoma data. This study provides a detailed meta-analysis of the literature on clinical outcomes, diagnosis, and treatment of patients with glioblastoma. We also share new and thoughtful insights into this disease in the era of precision medicine.
Materials and Methods
In this study, we searched the data using the Adjutant R suite [13] to achieve a more efficient and reproducible analysis process. We searched for published articles on glioblastoma, glioma pathophysiology, diagnosis, and treatment between January 1990 and February 2020. The database results include metadata: PubMed ID, publication year, author, article title, abstract, and Medical Subject Heading (MeSH) terms. Names and abstracts are split into a phrase and stemmed and filtered by the Adjutant package. Time frequency inverses the measured data frequency for each time period and creates a sparse document term matrix (DTM) for further analysis. t-distributed stochastic neighbor embedding (t-SNE) and hdbscan algorithms were used to perform unsupervised clustering using DTM data. The coordinates generated by t-SNE are used in hdbscan algorithm to provide topic clusters. Then assign a topic to each group using the top five statements in the group. Manual Selection: Inclusion and Exclusion Criteria
After topic grouping, we have our group use external guidance to evaluate journal content and discussion topics. All test items are reviewed and deemed suitable for further review or rejection. The inclusion criteria were the main points evaluated by neurosurgeons specializing in English-English; we continue to edit the body and group name. Supplementary Table S1 contains a list of all articles and their corresponding categories. Data analysis
Results
Data search and topic integration
The initial analysis was performed on a library of 2799 glioblastoma-related articles published in the last 30 years (Supplementary Table S1). We informally group topics using article names and descriptions and classify items according to their categories. Items that did not form part of the cluster were removed from further analysis, leaving 1314 records that formed 27 clusters. The topic group was determined using the five most prevalent categories in the group and the editorial articles included in the book (Figure 1). The full list of articles and related categories is provided in Supplementary Table S1.
Figure 1. Topic representation of the included literature related to glioblastoma. The figure highlights twenty-seven clusters.
Pathogenesis of Glioblastoma
High-Grade Glioma Factors
Risk factors for the development of glioblastoma are still unknown and research on this topic is often unreliable. Exposure to ionizing radiation during childhood to treat malignant tumors [15] is a low-risk factor for the development of glioma. The risk of developing a brain tumor after radiation therapy increases when radiation therapy is given at a younger age (<5 years) and appears to be a volume- and dose-dependent effect, but there is no clear indication of a threshold [16,17]. The increased incidence of glioblastoma [18,19] raises the issue of environmental risk. A meta-analysis reported an association between cell phone use and the development of glioblastoma [20]. However, these results are inconsistent and controversial in other studies [21,22]. The role of smoking or carcinogens has been investigated but has not been proven to be associated with glioblastoma [23,24]. In rare cases (<1%), patients with Lynch, Turcot type 1 or Li Fraumeni syndrome have a genetic predisposition to the development of glioblastoma [25]. Clinical Manifestations Clinical manifestations depend on the location and size of the tumor at diagnosis. The most common presentation at diagnosis is headache and/or nausea in the presence of a large or severe tumor. Symptoms related to intracranial hypertension accounted for 30% of symptoms, followed by physical weakness (20%), weight loss and pain (17%), confusion (15%), and visual or speech impairment (13%) [26]. Seizures are rare (15% to 20%) and can be easily controlled with anticonvulsant therapy. Seizures are associated with better vision, probably because the cortical location of glioblastoma is associated with seizures [27]. These symptoms are often confusing and lead to diagnosis weeks or months from onset. Figure 2 shows the clinical presentation of the diagnosis (<3%). Up to 25% of glioblastomas occur in the frontal lobe, the largest lobe of the brain [28], thus causing cognitive and functional impairment in 15% of patients [26].
Figure 2. Overview of the main reported clinical features in glioblastomas.
Radiological features
The magnetic resonance imaging (MRI) features of glioblastoma are well known [2,28-31]. They have infiltrative, heterogeneous intraparenchymal lesions that arise and spread from the white matter. Involvement of the corpus callosum is common. The margins of the glioblastoma are indistinct and there is increased contrast between the margins, a sign of damage to the blood vessels in the brain. Due to necrosis, the lesion area appears hypointense on T1-weighted images, while the lesion is surrounded by cerebral edema that appears hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images. Diffusion-weighted imaging and apparent diffusion coefficient can provide important information about the suspicion of malignancy in stellate cell tumors. New multimodal MR techniques, such as diffusion/perfusion sequences, provide more information about the characteristics of the pain itself and help to obtain more accurate results. Perfusion-weighted imaging (PWI) shows increased cerebral blood flow corresponding to neovascularization and blood-brain barrier disruption. MR spectroscopy features of glioblastoma are increased choline/N-acetylaspartate and choline/creatinine ratios. However, these features are related to cell function and are not specific enough to diagnose glioblastoma in isolation. In addition, increased lactate and lipid peaks and decreased peak myo-inositol are reliable data for the diagnosis of glioblastoma [30,32] and help distinguish glioblastoma from metastases, lymphomas and brain abscesses. As some authors have pointed out, these studies also help to define the effects of peritumoral invasion and can be used as a guide for biopsy [33,34] and to monitor changes in disease after treatment. Basic and Molecular Pathology
The diagnosis of glioblastoma can be easily established by surgical resection or biopsy. Glioblastoma is a grade IV glioma classified according to the central classification of brain tumors of the World Health Organization (WHO) [35,36]. Glioblastoma has poorly differentiated, often pleomorphic tumor cells and has a predominantly stellate cell differentiation [37]. Histopathological features include nuclear atypia, cellular pleomorphism, high mitotic activity, vascular thrombosis, microvascular proliferation, and necrosis [28]. Since the 2016 WHO classification of central nervous system tumors [36] and advances in immunohistochemistry, glioblastoma is now classified according to isocitrate dehydrogenase (IDH), with sites classified as glioblastoma IDH mutant or IDH wild type. The latter is by far the most common, accounting for 90% of patients, and is most commonly seen in patients over 55 years of age. Glioblastoma IDH mutations occur more frequently in younger patients (10% of patients), usually arise from low-grade glioma mutations, and are associated with longer survival [38]. Complementary immunohistochemistry and molecular techniques are now routinely used for diagnostic and prognostic purposes. (IDH) mutation and hypermethylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene promoter [39] predict longer survival, whereas telomerase reverse transcriptase promoter (TERTp) mutation and deletion of Chromosome 10 are negative. The significance of gain-of-function differences in the p53 gene [40] and epidermal growth factor receptor (EGFR) gene alterations [41] is still unknown. MGMT plays a role in DNA repair and its effects are associated with resistance to drugs such as temozolomide, the most commonly used first-line chemotherapy in glioblastoma. Therefore, hypermethylation of the MGMT gene promoter is an important prognostic marker and is associated with better and longer progression-free survival [42]. Recently, mutations in the tumor suppressor gene phosphatase and tensin homolog (PTEN) or deletions on chromosome 10 have been shown to be associated with the development of glioblastoma [43]. Current management
1.2.1. Surgical procedures
Whenever possible, the first step is to complete macroscopic surgical excision. Literature suggests that excision of >90% of the difference among uncomplicated patients improves patient outcome and recurrence [44–46]. Surgical resection is generally recommended for patients under 70 years of age, in good condition (Karnofsky score >70) [46] and in whom the tumor can be resected. Otherwise, surgical debulking or stereotaxic biopsy is performed to confirm the diagnosis before further treatment [47,48]. Because of the importance of complete resection for survival, advances have been made in surgical techniques such as awake craniotomy or neuromonitoring [51] to improve the quality of the cut and prevent defects [49,50]. In addition, fluorescence-guided surgery has been developed to guide surgery, resulting in improved outcomes and improved survival [52–54]. Recently, ablation equipment has improved with the use of laser interstitial thermal therapy (LITT) [55], which allows for less percutaneous intervention through the insertion of an optical fiber. The resulting thermal damage causes tumor necrosis [55–57]. Another new aspect of surgical treatment of brain tumors is the intraoperative monitoring of tumor metabolites based on mass spectrometry. Analysis of cellular contents allows accurate molecular identification of tumors and visualization of tumor resection [58,59]. Figure 3 shows clinical outcomes, radiation, disease, treatment, and their interactions.
Figure 3. Integrative visualization summary of main clinical symptoms and signs and their interactions with radiology, biology and treatment features. The box and annotation sizes are proportional to the item frequency. Clinical: blue, Biology: purple, Radiology: green, Treatment: orange.
Medical Treatment of Glioblastomas
The standard of care for patients aged less than 70 relies on radiotherapy (RT) and adjuvant temozolomide. This protocol improved the overall survival in a large randomized phase III trial [60]. Radiotherapy is given for a six-week period with a total dose of 60 grays. Temozolomide is an alkylating agent administered daily during the RT and then, for six cycles of five consecutive days per month, one month after the end of the RT. The absence of hypermethylated MGMT promoter [61] is a negative prognostic and predictive factor of temozolomide efficiency. The treatment protocol proposed by Stupp allows for increasing the average survival rate from 12.1 months using RT alone to 14.6 months, and the two-year survival rate from 8 to 26% with concomitant temozolomide [60]. This randomized controlled trial did not include patients older than 70. In the latter population, the standard of care is based on hypofractionated radiotherapy and temozolomide [62] whenever feasible, but the treatment depends on the patient’s general condition. RT alone (54 grays) has been proposed with a positive impact on survival (29.1 weeks compared to 16.9 weeks in patients with supportive care alone) and with no alteration of the quality of life [63]. These results have been validated in patients with a Karnofsky [64] performance status (KPS) > 60. More recently, some authors have shown that short-course RT plus temozolomide was associated with longer survival (9.3 versus 7.6 months) in older patients (>65 years). Malmstrom et al. [65] randomized patients aged 60 years and older presenting with a glioblastoma to assess the optimal palliative treatment. The conclusion of the trial was that radiotherapy alone is associated with poor outcomes. On the contrary, both temozolamide and hypofractionated radiotherapy appeared as standards of care especially in patients with methylation of the MGMT gene promoter. In patients with poor general health, supportive care may be proposed to preserve the quality of life with the shortest length of hospital stay if possible [62].
The disease progression is evaluated using brain MRI every 2 to 3 months according to the response assessment in Neuro-oncology (RANO) criteria [66]. At the time of recurrence, there is no standard of care. The main determinants for treatment proposals are the patients’ general condition and treatments previously administered [67]. A second surgery can be proposed in young patients with preserved KPS. This strategy has been shown to be associated with longer survival in selected patients [68] (14 months versus 22 months of overall survival in patients with second surgery at recurrence). Eighty percent of patients did not need rehabilitation after a second surgery. In selected patients, the use of intracavitary carmustine wafers (BCNU) has been proposed at initial surgery or in case of recurrence [69]. Efficiency and adverse effects of this therapy remains a matter of debate [70,71]. In the absence of safe possible resection, a second line chemotherapy can be proposed including nitrosoureas, temozolomide or antiangiogenic drugs such as bevacizumab but with no clear results in terms of benefit to date [72] and poor outcomes with an average overall survival rate of 6 months from recurrence [73]. Due to the poor prognosis of glioblastomas, there is an urgent need for new therapies. The REGOMA trial (Regorafenib in Relapsed Glioblastoma) [74] is a phase II randomized trial. The aim was to propose the use regorafenib, an inhibitor of angiogenic and oncogenic receptor of tyrosine kinases at the recurrence of glioblastomas. The results were rather positive on overall survival and with few side effects. A phase III trial is expected.
Current Research and Perspectives
Omics Approaches
Over the last decade, precision medicine, also through omics approaches (Figure 4), has offered new insights in the diagnosis and management of glioblastomas [75,76]. In the past few years, radiomics brought significant insights in the characterization and predictive models of glioblastomas [77,78]. Radiomics is based on the extraction of a large amount of data from medical images. Radiomics is then enriched with clinical, genomics and proteomics data to establish new diagnosis and prognosis criteria to enhance treatment efficiency. Other promising areas in the fight against glioblastoma are the genomics and transcriptomics approaches. The emergence of big data in the precision medicine offered new therapeutic perspectives. Through ambitious projects such as the human genome project [79], genomics helped us in improving the understanding of glioblastoma. In the continuation of the project, RNA sequencing-based genes proposed genomics signature of life expectancy in patients with glioblastomas [80]. Transcriptomics and other omics technics proposed prognostic tools for the comprehension of the disease [81,82]. Along with these techniques, liquid chromatography and mass spectrometry analyses from different samples such as CSF (Cerebrospinal fluid), urine, blood or glioblastoma cell lines [83] have provided a comprehensive view of the altered metabolic pathways in patients with glioblastoma [84,85]. More recently, special attention has been given to alterations of lipid metabolism in glioblastomas. Based on omics human studies, Guo et al. have described a decrease of 90% of lipid levels in tumor tissue except for phosphatidylcholine and cholesterol ester levels which appeared high in glioma tissue while they are absent in normal brain tissue [86]. These data may emphasize the key role of certain lipids in the glioblastoma metabolism to facilitate tumor growth. Moreover, the metabolic signature of brain tumors in the plasma is of interest for the grading and prognosis of these diseases. It has been shown that the plasma level of metabolites of interest can help to define the grading of brain glioma and to provide prognostic information in patients with a similar glioma grade [87]. Nevertheless, data are still rare for these emerging approaches; however, the promise of precision medicine and the surge of multimodal data-driven strategies can provide valuable tools for the development of biomarkers and innovative therapies in glioblastomas [88].
Novel Therapies for Glioblastomas
Due to the adverse outcome in patients with glioblastoma and the high frequency of this disease, innovative therapies are being tested in different randomized controlled trials [73,89,90]. With the development of a better understanding of molecular pathways triggering glioblastoma growth [27–29], the traditional approach of antitumor therapy is being progressively complemented by a more personalized approach [91,92]. Treatment schedules have been rethought as well as the drugs themselves. Two major drawbacks consist in the difficulty for most of the drugs is to pass through the blood–brain barrier (BBB) and to target tumor cells due to the presence of abnormal vessels and necrosis, which hampers drugs being delivered at a suitable concentration. Some emerging techniques have been proposed to improve the distribution of antitumor therapy, notably the conjugation of drugs with protein to facilitate the movement across the BBB and specifically target the tumor [93], the use of convection-enhanced delivery consisting in the direct administration inside the tumor via a catheter [94] and the use of nanoparticles. The increase in BBB permeability during chemotherapy administration via focused ultrasounds is being also tested [95]. Meanwhile, immunotherapy approaches are known for a long time, with promising results in many cancers such as melanoma but deceiving results in patients with gliomas [96]. Cancer vaccination has recently been proposed referring to the activation of an immune response against tumor antigens. These new technologies have been applied for glioblastoma treatment with different vectors [97–99]. Two modalities have been tested: peptide vaccines targeting EGFR, IDH1 or heat shock proteins, and cell-based vaccines consisting in the injection of ex vivo modified cells, mainly dendritic cells [100]. Despite encouraging results in animal models in terms of disease control [101], cancer vaccination in glioblastomas has not yet proven its efficacy on overall survival in phase III studies [9]. Recently, the role of tumor-associated macrophages (TAM) has been highlighted in the genesis and resistance to treatment of glioblastoma cells [102–104]. Landry et al. [102] showed that the TAM located in the core have different characteristics and metabolic pathways compared to those located in the periphery of the glioblastoma. For these reasons, they reaffirm the need for a multi-targeted approach through a modulation of the TAM. Furthermore, tumor-associated neutrophils (TAN) are found to be involved in necrosis onset in glioblastoma patients [105,106]. In this context, the mechanism of necrosis could be a neutrophil-mediated ferroptosis. The latter could have a pro-tumorigenic role [105]. Thus, targeted therapies are potential novel therapies for glioblastomas to prevent TAN recruitment. Stupp et al. recently developed a new therapeutic modality in the treatment of recurrent glioblastomas consisting in the local delivery of low-intensity electric fields via a non-invasive transducer [73]. The device (NovoTTF-100A) was tested in a phase III study and randomized with active chemotherapy as an alternative arm. The overall survival was similar in both arms (6 months) with fewer adverse events in the NovoTTF-100A group and a better quality of life. For this reason, the tumor-treating field is considered as a standard of care in some guidelines [107]. Moreover, in the last years, the association of molecularly targeted drugs such as tyrosine kinase administration or others combined with X-rays to decrease radioresistance due to hypoxia showed encouraging results [108–110].
Figure 4. Overview of the main driving omics technologies and therapeutic perspectives for glioblastoma in the precision medicine era. This figure has been created with BioRender.com (accessed on 9 March 2021).
Conclusions
The authors are aware of the limitations of using such technology to search for information. This limitation may be related to the up-to-dateness or scope of such equipment and may result in some important information being lost. This highlights the importance of manual therapy combined with the use of multiple tools to help address areas of interest. Survival outcomes remain poor. Many attempts are now being made with new treatments that are shifting from a universal approach to a more personalized approach. The main purpose of this procedure is to deliver selected drugs to the tumor and adjust their concentration according to tumor characteristics. From now on, the process of delivering the drug will be as difficult as the drug itself.
References
1. Bauchet, L.; Ostrom, Q.T. Epidemiology and Molecular Epidemiology. Neurosurg. Clin. N. Am. 2019, 30, 1–16. [CrossRef]
2. Negendank, W.G.; Sauter, R.; Brown, T.R.; Evelhoch, J.L.; Falini, A.; Gotsis, E.D.; Heerschap, A.; Kamada, K.; Lee, B.C.; Mengeot, M.M.; et al. Proton magnetic resonance spectroscopy in patients with glial tumors: A multicenter study. J. Neurosurg. 1996, 84, 449–458. [CrossRef]
3. Morgan, L.L. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2015, 17, 623–624. [CrossRef]
4. Kowalczyk, T.; Ciborowski, M.; Kisluk, J.; Kretowski, A.; Barbas, C. Mass spectrometry based proteomics and metabolomics in personalized oncology. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165690. [CrossRef]
5. Dolecek, T.A.; Propp, J.M.; Stroup, N.E.; Kruchko, C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012, 14 (Suppl. 5), v1–v49. [CrossRef]
6. Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of the science” review. Neuro Oncol. 2014, 16, 896–913. [CrossRef]
7. Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [CrossRef]
8. Touat, M.; Idbaih, A.; Sanson, M.; Ligon, K.L. Glioblastoma targeted therapy: Updated approaches from recent biological insights. Ann. Oncol. 2017, 28, 1457–1472. [CrossRef] [PubMed]
9. Malkki, H. Trial Watch: Glioblastoma vaccine therapy disappointment in Phase III trial. Nat. Rev. Neurol. 2016, 12, 190. [CrossRef] [PubMed]
10. Delgado-Lopez, P.D.; Corrales-Garcia, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [CrossRef] [PubMed]
11. Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [CrossRef]
12. Lopez de Maturana, E.; Alonso, L.; Alarcon, P.; Martin-Antoniano, I.A.; Pineda, S.; Piorno, L.; Calle, M.L.; Malats, N. Challenges in the Integration of Omics and Non-Omics Data. Genes 2019, 10, 238. [CrossRef] [PubMed]
13. Crisan, A.; Munzner, T.; Gardy, J.L.; Wren, J. Adjutant: An R-based tool to support topic discovery for systematic and literature reviews. Bioinformatics 2019, 35, 1070–1072. [CrossRef] [PubMed]
14. R Core Team. A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2020.
15. Connelly, J.M.; Malkin, M.G. Environmental risk factors for brain tumors. Curr. Neurol. Neurosci. Rep. 2007, 7, 208–214. [CrossRef] [PubMed]
16. Wingren, C.; James, P.; Borrebaeck, C.A.K. Strategy for surveying the proteome using affinity proteomics and mass spectrometry.
Proteomics 2009, 9, 1511–1517. [CrossRef]
17. Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-induced gliomas: A comprehensive review and meta-analysis. Neurosurg. Rev. 2018, 41, 719–731. [CrossRef]
18. Philips, A.; Henshaw, D.L.; Lamburn, G.; O’Carroll, M.J. Brain Tumours: Rise in Glioblastoma Multiforme Incidence in England 1995-2015 Suggests an Adverse Environmental or Lifestyle Factor. J. Environ. Public Health 2018, 2018, 7910754. [CrossRef]
19. Dobes, M.; Khurana, V.G.; Shadbolt, B.; Jain, S.; Smith, S.F.; Smee, R.; Dexter, M.; Cook, R. Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000–2008): Findings of a multicenter Australian study. Surg. Neurol. Int. 2011, 2, 176. [CrossRef]
20. Yang, M.; Guo, W.; Yang, C.; Tang, J.; Huang, Q.; Feng, S.; Jiang, A.; Xu, X.; Jiang, G. Mobile phone use and glioma risk: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175136. [CrossRef]
21. Karipidis, K.; Elwood, M.; Benke, G.; Sanagou, M.; Tjong, L.; Croft, R.J. Mobile phone use and incidence of brain tumour histological types, grading or anatomical location: A population-based ecological study. BMJ Open 2018, 8, e024489. [CrossRef]
22. Repacholi, M.H.; Lerchl, A.; Roosli, M.; Sienkiewicz, Z.; Auvinen, A.; Breckenkamp, J.; d’Inzeo, G.; Elliott, P.; Frei, P.; Heinrich, S.; et al. Systematic review of wireless phone use and brain cancer and other head tumors. Bioelectromagnetics 2012, 33, 187–206. [CrossRef]
23. Benke, G.; Turner, M.C.; Fleming, S.; Figuerola, J.; Kincl, L.; Richardson, L.; Blettner, M.; Hours, M.; Krewski, D.; McLean, D.; et al. Occupational solvent exposure and risk of glioma in the INTEROCC study. Br. J. Cancer 2017, 117, 1246–1254. [CrossRef]
24. Parent, M.E.; Turner, M.C.; Lavoue, J.; Richard, H.; Figuerola, J.; Kincl, L.; Richardson, L.; Benke, G.; Blettner, M.; Fleming, S.; et al. Lifetime occupational exposure to metals and welding fumes, and risk of glioma: A 7-country population-based case-control study. Environ. Health 2017, 16, 90. [CrossRef] [PubMed]
25. Rice, T.; Lachance, D.H.; Molinaro, A.M.; Eckel-Passow, J.E.; Walsh, K.M.; Barnholtz-Sloan, J.; Ostrom, Q.T.; Francis, S.S.; Wiemels, J.; Jenkins, R.B.; et al. Understanding inherited genetic risk of adult glioma—A review. Neurooncol. Pract. 2016, 3, 10–16. [CrossRef] [PubMed]
26. Yuile, P.; Dent, O.; Cook, R.; Biggs, M.; Little, N. Survival of glioblastoma patients related to presenting symptoms, brain site and treatment variables. J. Clin. Neurosci. 2006, 13, 747–751. [CrossRef]
27. Vecht, C.J.; Kerkhof, M.; Duran-Pena, A. Seizure prognosis in brain tumors: New insights and evidence-based management. Oncologist 2014, 19, 751–759. [CrossRef] [PubMed]
28. Wirsching, H.G.; Galanis, E.; Weller, M. Glioblastoma. Handb. Clin. Neurol. 2016, 134, 381–397. [CrossRef] [PubMed]
29. Yan, J.L.; Li, C.; Boonzaier, N.R.; Fountain, D.M.; Larkin, T.J.; Matys, T.; van der Hoorn, A.; Price, S.J. Multimodal MRI characteristics of the glioblastoma infiltration beyond contrast enhancement. Ther. Adv. Neurol. Disord. 2019, 12. [CrossRef] [PubMed]
30. Peeken, J.C.; Goldberg, T.; Pyka, T.; Bernhofer, M.; Wiestler, B.; Kessel, K.A.; Tafti, P.D.; Nusslin, F.; Braun, A.E.; Zimmer, C.; et al. Combining multimodal imaging and treatment features improves machine learning-based prognostic assessment in patients with glioblastoma multiforme. Cancer Med. 2019, 8, 128–136. [CrossRef]
31. Law, M.; Yang, S.; Wang, H.; Babb, J.S.; Johnson, G.; Cha, S.; Knopp, E.A.; Zagzag, D. Glioma grading: Sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 2003, 24, 1989–1998.
32. Price, S.J.; Young, A.M.; Scotton, W.J.; Ching, J.; Mohsen, L.A.; Boonzaier, N.R.; Lupson, V.C.; Griffiths, J.R.; McLean, M.A.; Larkin,
T.J. Multimodal MRI can identify perfusion and metabolic changes in the invasive margin of glioblastomas. J. Magn. Reson. Imaging 2016, 43, 487–494. [CrossRef]
33. Barajas, R.F., Jr.; Hodgson, J.G.; Chang, J.S.; Vandenberg, S.R.; Yeh, R.F.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Dillon, W.P.; Cha, S. Glioblastoma multiforme regional genetic and cellular expression patterns: Influence on anatomic and physiologic MR imaging. Radiology 2010, 254, 564–576. [CrossRef]
34. Lonjon, M.; Mondot, L.; Lonjon, N.; Chanalet, S. Clinical factors in glioblastoma and neuroradiology. Neurochirurgie 2010, 56, 449–454. [CrossRef]
35. Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [CrossRef]
36. Louis, O.H.; Wiestler, O.D.; Cavenee, W.K. The 2016 World Health Organization Classification of Tumors of the Central Nervous System, 4th ed.; IARC Publication: Geneva, Switzerland, 2016; Volume 1.
37. Figarella-Branger, D.; Bouvier, C.; Moroch, J.; Michalak, S.; Burel-Vandenbos, F. Morphological classification of glioblastomas. Neurochirurgie 2010, 56, 459–463. [CrossRef]
38. Banan, R.; Hartmann, C. The new WHO 2016 classification of brain tumors-what neurosurgeons need to know. Acta Neurochir. 2017, 159, 403–418. [CrossRef]
39. Zhao, Y.H.; Wang, Z.F.; Cao, C.J.; Weng, H.; Xu, C.S.; Li, K.; Li, J.L.; Lan, J.; Zeng, X.T.; Li, Z.Q. The Clinical Significance of O(6)-Methylguanine-DNA Methyltransferase Promoter Methylation Status in Adult Patients With Glioblastoma: A Meta-analysis. Front. Neurol. 2018, 9, 127. [CrossRef]
40. Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [CrossRef] [PubMed]
41. Eskilsson, E.; Rosland, G.V.; Solecki, G.; Wang, Q.; Harter, P.N.; Graziani, G.; Verhaak, R.G.W.; Winkler, F.; Bjerkvig, R.; Miletic, H. EGFR heterogeneity and implications for therapeutic intervention in glioblastoma. Neuro Oncol. 2018, 20, 743–752. [CrossRef] [PubMed]
42. Binabaj, M.M.; Bahrami, A.; ShahidSales, S.; Joodi, M.; Joudi Mashhad, M.; Hassanian, S.M.; Anvari, K.; Avan, A. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J. Cell Physiol. 2018, 233, 378–386. [CrossRef] [PubMed]
43. Benitez, J.A.; Ma, J.; D’Antonio, M.; Boyer, A.; Camargo, M.F.; Zanca, C.; Kelly, S.; Khodadadi-Jamayran, A.; Jameson, N.M.; Andersen, M.; et al. PTEN regulates glioblastoma oncogenesis through chromatin-associated complexes of DAXX and histone H3.3. Nat. Commun. 2017, 8, 15223. [CrossRef] [PubMed]
44. Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [CrossRef] [PubMed]
45. Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [CrossRef] [PubMed]
46. Chambless, L.B.; Kistka, H.M.; Parker, S.L.; Hassam-Malani, L.; McGirt, M.J.; Thompson, R.C. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J. Neurooncol. 2015, 121, 359–364. [CrossRef] [PubMed]
47. Marcus, H.J.; Vakharia, V.N.; Ourselin, S.; Duncan, J.; Tisdall, M.; Aquilina, K. Robot-assisted stereotactic brain biopsy: Systematic review and bibliometric analysis. Childs Nerv. Syst. 2018, 34, 1299–1309. [CrossRef]
48. McGirt, M.J.; Villavicencio, A.T.; Bulsara, K.R.; Friedman, A.H. MRI-guided stereotactic biopsy in the diagnosis of glioma: Comparison of biopsy and surgical resection specimen. Surg. Neurol. 2003, 59, 279–283. [CrossRef]
49. Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Lee, Y.M.; Nangiana, J.; Vivas-Buitrago, T.; Quinones-Hinojosa, A. Awake Craniotomy vs Craniotomy Under General Anesthesia for Perirolandic Gliomas: Evaluating Perioperative Complications and Extent of Resection. Neurosurgery 2017, 81, 481–489. [CrossRef]
50. Foster, C.H.; Morone, P.J.; Cohen-Gadol, A. Awake craniotomy in glioma surgery: Is it necessary? J. Neurosurg. Sci. 2019, 63, 162–178. [CrossRef]
51. Obermueller, T.; Schaeffner, M.; Shiban, E.; Droese, D.; Negwer, C.; Meyer, B.; Ringel, F.; Krieg, S.M. Intraoperative neuromonitor- ing for function-guided resection differs for supratentorial motor eloquent gliomas and metastases. BMC Neurol. 2015, 15, 211. [CrossRef]
52. Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [CrossRef]
53. Cho, S.S.; Salinas, R.; Lee, J.Y.K. Indocyanine-Green for Fluorescence-Guided Surgery of Brain Tumors: Evidence, Techniques, and Practical Experience. Front. Surg. 2019, 6, 11. [CrossRef] [PubMed]
54. Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5- aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [CrossRef]
55. Norred, S.E.; Johnson, J.A. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: A review.
BioMed Res. Int. 2014, 2014, 761312. [CrossRef] [PubMed]
56. Kamath, A.A.; Friedman, D.D.; Akbari, S.H.A.; Kim, A.H.; Tao, Y.; Luo, J.; Leuthardt, E.C. Glioblastoma Treated With Magnetic Resonance Imaging-Guided Laser Interstitial Thermal Therapy: Safety, Efficacy, and Outcomes. Neurosurgery 2019, 84, 836–843. [CrossRef] [PubMed]
57. Carpentier, A.; Chauvet, D.; Reina, V.; Beccaria, K.; Leclerq, D.; McNichols, R.J.; Gowda, A.; Cornu, P.; Delattre, J.Y. MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg. Med. 2012, 44, 361–368. [CrossRef] [PubMed]
58. Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [CrossRef]
59. Santagata, S.; Eberlin, L.S.; Norton, I.; Calligaris, D.; Feldman, D.R.; Ide, J.L.; Liu, X.; Wiley, J.S.; Vestal, M.L.; Ramkissoon, S.H.; et al. Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc. Natl. Acad. Sci. USA 2014, 111, 11121–11126. [CrossRef]
60. Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [CrossRef]
61. Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; de Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [CrossRef] [PubMed]
62. Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [CrossRef]
63. Keime-Guibert, F.; Chinot, O.; Taillandier, L.; Cartalat-Carel, S.; Frenay, M.; Kantor, G.; Guillamo, J.S.; Jadaud, E.; Colin, P.; Bondiau, P.Y.; et al. Radiotherapy for glioblastoma in the elderly. N. Engl. J. Med. 2007, 356, 1527–1535. [CrossRef] [PubMed]
64. Mor, V.; Laliberte, L.; Morris, J.N.; Wiemann, M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984, 53, 2002–2007. [CrossRef]
65. Malmstrom, A.; Gronberg, B.H.; Marosi, C.; Stupp, R.; Frappaz, D.; Schultz, H.; Abacioglu, U.; Tavelin, B.; Lhermitte, B.; Hegi, M.E.; et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: The Nordic randomised, phase 3 trial. Lancet Oncol. 2012, 13, 916–926. [CrossRef]
66. Chukwueke, U.N.; Wen, P.Y. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019, 8, CNS28. [CrossRef]
67. Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. 2013, 15, 4–27. [CrossRef] [PubMed]
68. Wann, A.; Tully, P.A.; Barnes, E.H.; Lwin, Z.; Jeffree, R.; Drummond, K.J.; Gan, H.; Khasraw, M. Outcomes after second surgery for recurrent glioblastoma: A retrospective case-control study. J. Neurooncol. 2018, 137, 409–415. [CrossRef]
69. Xing, W.K.; Shao, C.; Qi, Z.Y.; Yang, C.; Wang, Z. The role of Gliadel wafers in the treatment of newly diagnosed GBM: A meta-analysis. Drug Des. Dev. Ther. 2015, 9, 3341–3348. [CrossRef]
70. Sage, W.; Guilfoyle, M.; Luney, C.; Young, A.; Sinha, R.; Sgubin, D.; McAbee, J.H.; Ma, R.; Jefferies, S.; Jena, R.; et al. Local alkylating chemotherapy applied immediately after 5-ALA guided resection of glioblastoma does not provide additional benefit.
J. Neurooncol. 2018, 136, 273–280. [CrossRef]
71. Grangeon, L.; Ferracci, F.X.; Fetter, D.; Maltete, D.; Langlois, O.; Gilard, V. How safe are carmustine wafers? Rev. Neurol. 2018, 174, 346–351. [CrossRef]
72. Taal, W.; Oosterkamp, H.M.; Walenkamp, A.M.E.; Dubbink, H.J.; Beerepoot, L.V.; Hanse, M.C.J.; Buter, J.; Honkoop, A.H.; Boerman, D.; de Vos, F.Y.F.; et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014, 15, 943–953. [CrossRef]
73. Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [CrossRef] [PubMed]
74. Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Ruda, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F.; et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [CrossRef]
75. Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and Therapeutic Biomarkers in Glioblastoma: Current Status and Future Perspectives. BioMed Res. Int. 2017, 2017, 8013575. [CrossRef]
76. Sasmita, A.O.; Wong, Y.P.; Ling, A.P.K. Biomarkers and therapeutic advances in glioblastoma multiforme. Asia Pac. J. Clin. Oncol.
2018, 14, 40–51. [CrossRef] [PubMed]
77. Chaddad, A.; Daniel, P.; Desrosiers, C.; Toews, M.; Abdulkarim, B. Novel Radiomic Features Based on Joint Intensity Matrices for Predicting Glioblastoma Patient Survival Time. IEEE J. Biomed. Health Inform. 2019, 23, 795–804. [CrossRef] [PubMed]
78. Chaddad, A.; Daniel, P.; Sabri, S.; Desrosiers, C.; Abdulkarim, B. Integration of Radiomic and Multi-omic Analyses Predicts Survival of Newly Diagnosed IDH1 Wild-Type Glioblastoma. Cancers 2019, 11, 1148. [CrossRef]
79. Hood, L.; Rowen, L. The Human Genome Project: Big science transforms biology and medicine. Genome Med. 2013, 5, 79. [CrossRef] [PubMed]
80. Zuo, S.; Zhang, X.; Wang, L. A RNA sequencing-based six-gene signature for survival prediction in patients with glioblastoma. Sci. Rep. 2019, 9, 2615. [CrossRef]
81. Lin, W.; Huang, Z.; Xu, Y.; Chen, X.; Chen, T.; Ye, Y.; Ding, J.; Chen, Z.; Chen, L.; Qiu, X.; et al. A three-lncRNA signature predicts clinical outcomes in low-grade glioma patients after radiotherapy. Aging 2020, 12, 9188–9204. [CrossRef] [PubMed]
82. Stackhouse, C.T.; Gillespie, G.Y.; Willey, C.D. Exploring the Roles of lncRNAs in GBM Pathophysiology and Their Therapeutic Potential. Cells 2020, 9, 2369. [CrossRef]
83. Marziali, G.; Signore, M.; Buccarelli, M.; Grande, S.; Palma, A.; Biffoni, M.; Rosi, A.; D’Alessandris, Q.G.; Martini, M.; Larocca, L.M.; et al. Metabolic/Proteomic Signature Defines Two Glioblastoma Subtypes With Different Clinical Outcome. Sci. Rep. 2016, 6, 21557. [CrossRef]
84. Zhai, X.H.; Xiao, J.; Yu, J.K.; Sun, H.; Zheng, S. Novel sphingomyelin biomarkers for brain glioma and associated regulation research on the PI3K/Akt signaling pathway. Oncol. Lett. 2019, 18, 6207–6213. [CrossRef] [PubMed]
85. Heiland, D.H.; Haaker, G.; Watzlawick, R.; Delev, D.; Masalha, W.; Franco, P.; Machein, M.; Staszewski, O.; Oelhke, O.; Nicolay, N.H.; et al. One decade of glioblastoma multiforme surgery in 342 elderly patients: What have we learned? J. Neurooncol. 2018, 140, 385–391. [CrossRef] [PubMed]
86. Guo, D.; Bell, E.H.; Chakravarti, A. Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol.
2013, 2, 289–299. [CrossRef] [PubMed]
87. Moren, L.; Bergenheim, A.T.; Ghasimi, S.; Brannstrom, T.; Johansson, M.; Antti, H. Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites 2015, 5, 502–520. [CrossRef]
88. Petralia, F.; Tignor, N.; Reva, B.; Koptyra, M.; Chowdhury, S.; Rykunov, D.; Krek, A.; Ma, W.; Zhu, Y.; Ji, J.; et al. Integrated Proteogenomic Characterization across Major Histological Types of Pediatric Brain Cancer. Cell 2020. [CrossRef]
89. Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [CrossRef]
90. Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F.; et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [CrossRef]
91. Jain, K.K. A Critical Overview of Targeted Therapies for Glioblastoma. Front. Oncol. 2018, 8, 419. [CrossRef]
92. Ene, C.I.; Holland, E.C. Personalized medicine for gliomas. Surg. Neurol. Int. 2015, 6, S89–S95. [CrossRef]
93. Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462. [CrossRef] [PubMed]
94. Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [CrossRef] [PubMed]
95. Parodi, A.; Rudzin´ska, M.; Deviatkin, A.; Soond, S.; Baldin, A.; Zamyatnin, A. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [CrossRef]
96. Franklin, C.; Livingstone, E.; Roesch, A.; Schilling, B.; Schadendorf, D. Immunotherapy in melanoma: Recent advances and future directions. Eur. J. Surg. Oncol. 2017, 43, 604–611. [CrossRef] [PubMed]
97. Kong, Z.; Wang, Y.; Ma, W. Vaccination in the immunotherapy of glioblastoma. Hum. Vaccines Immunother. 2018, 14, 255–268.
[CrossRef]
98. Kong, D.S.; Nam, D.H.; Kang, S.H.; Lee, J.W.; Chang, J.H.; Kim, J.H.; Lim, Y.J.; Koh, Y.C.; Chung, Y.G.; Kim, J.M.; et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget 2017, 8, 7003–7013. [CrossRef]
99. Swartz, A.M.; Batich, K.A.; Fecci, P.E.; Sampson, J.H. Peptide vaccines for the treatment of glioblastoma. J. Neurooncol. 2015, 123, 433–440. [CrossRef]
100. Eagles, M.E.; Nassiri, F.; Badhiwala, J.H.; Suppiah, S.; Almenawer, S.A.; Zadeh, G.; Aldape, K.D. Dendritic cell vaccines for high-grade gliomas. Ther. Clin. Risk Manag. 2018, 14, 1299–1313. [CrossRef]
101. Mitchell, D.A.; Batich, K.A.; Gunn, M.D.; Huang, M.N.; Sanchez-Perez, L.; Nair, S.K.; Congdon, K.L.; Reap, E.A.; Archer, G.E.; Desjardins, A.; et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 2015, 519, 366–369. [CrossRef]
102. Landry, A.P.; Balas, M.; Alli, S.; Spears, J.; Zador, Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci. Rep. 2020, 10, 19542. [CrossRef]
103. Gregoire, H.; Roncali, L.; Rousseau, A.; Cherel, M.; Delneste, Y.; Jeannin, P.; Hindre, F.; Garcion, E. Targeting Tumor Associated Macrophages to Overcome Conventional Treatment Resistance in Glioblastoma. Front. Pharmacol. 2020, 11, 368. [CrossRef]
104. Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.C.; Gutierrez-Vazquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [CrossRef] [PubMed]
105. Yee, P.P.; Wei, Y.; Kim, S.Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020, 11, 5424. [CrossRef]
106. Wang, T.; Cao, L.; Dong, X.; Wu, F.; De, W.; Huang, L.; Wan, Q. LINC01116 promotes tumor proliferation and neutrophil recruitment via DDX5-mediated regulation of IL-1beta in glioma cell. Cell Death Dis. 2020, 11, 302. [CrossRef] [PubMed]
107. Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [CrossRef]
108. Cammarata, F.P.; Torrisi, F.; Forte, G.I.; Minafra, L.; Bravata, V.; Pisciotta, P.; Savoca, G.; Calvaruso, M.; Petringa, G.; Cirrone, G.A.P.; et al. Proton Therapy and Src Family Kinase Inhibitor Combined Treatments on U87 Human Glioblastoma Multiforme Cell Line. Int. J. Mol. Sci. 2019, 20, 4745. [CrossRef]
109. Torrisi, F.; Minafra, L.; Cammarata, F.P.; Savoca, G.; Calvaruso, M.; Vicario, N.; Maccari, L.; Peres, E.A.; Ozcelik, H.; Bernaudin, M.; et al. SRC Tyrosine Kinase Inhibitor and X-rays Combined Effect on Glioblastoma Cell Lines. Int. J. Mol. Sci. 2020, 21, 3917. [CrossRef]
110. Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [CrossRef] [PubMed]
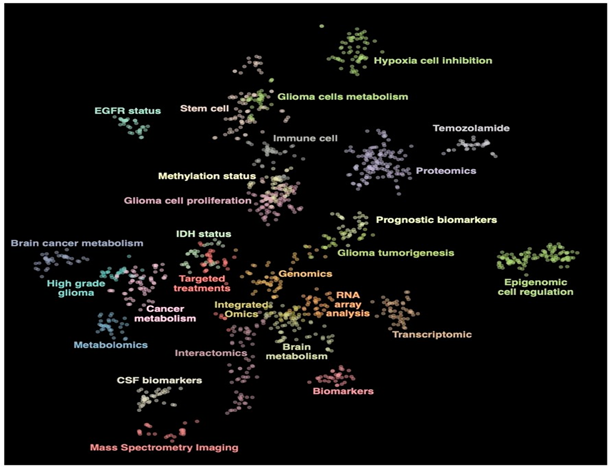
Figure 1
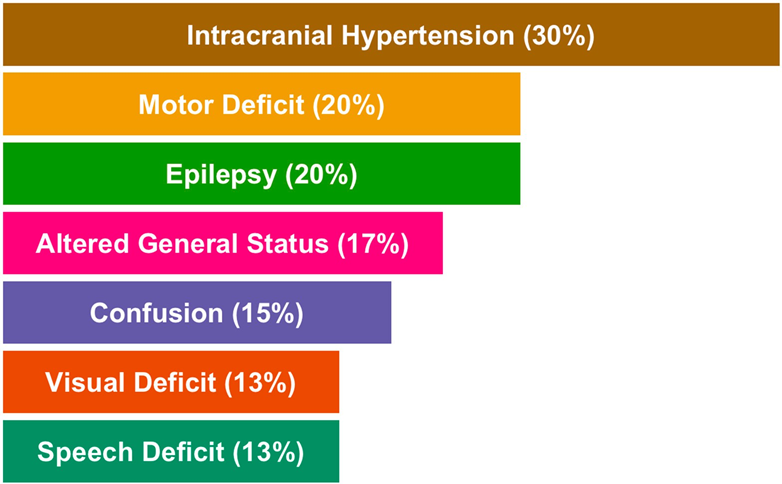
Figure 2
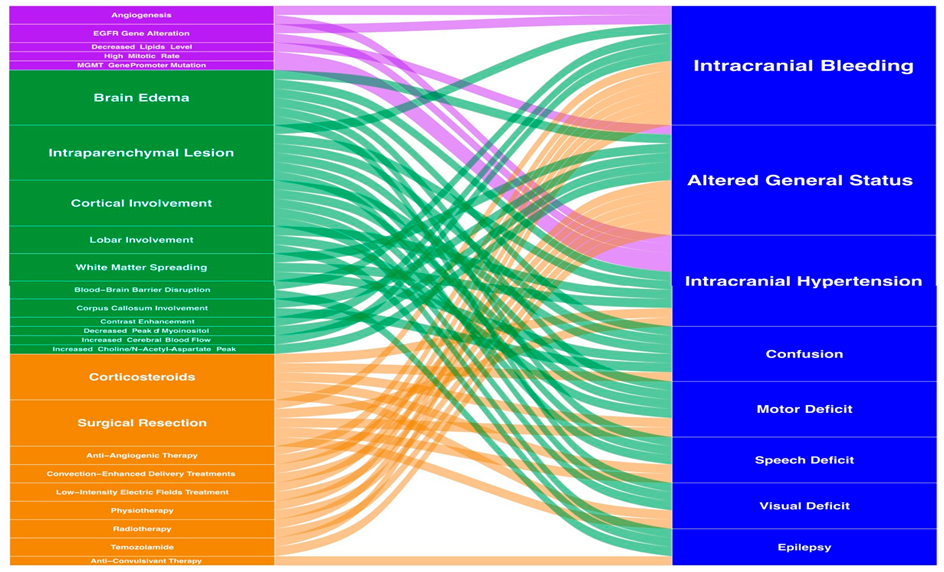
Figure 3
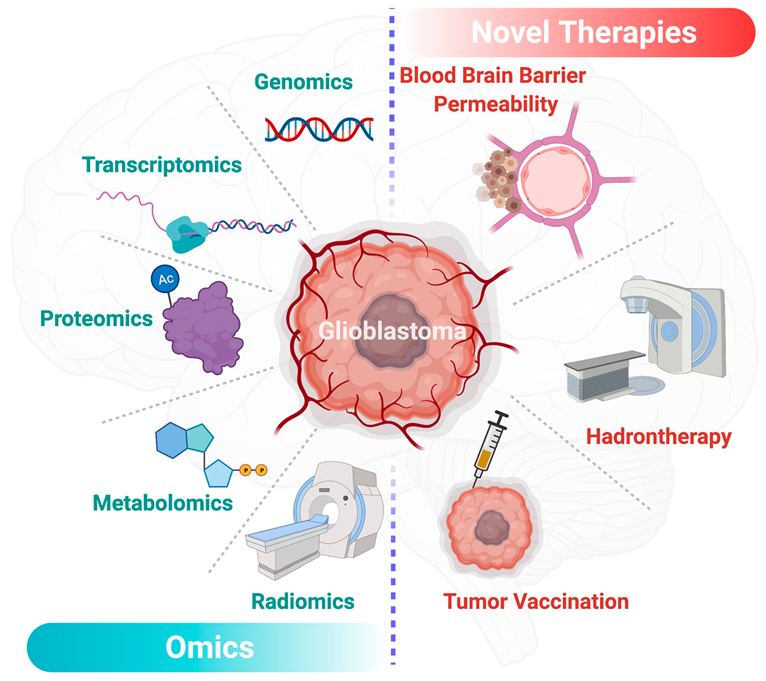
Figure 4
